��Ŀ����
I����1��CH3OH��1����H2��ȼ���ȷֱ�Ϊ��725.5 kJ/mol��285.8 kJ/mol��д����ҵ����CO2��H2�ϳ�CH3OH��1�����Ȼ�ѧ����ʽ�� ��
��2����ͼΪ����ˮ��װ�ã�д������ʱ�뵼��缫���淢���ĵ缫��Ӧʽ________��ĿǰӦ�����İ뵼�������Si����������������ǡ�21���͵���Դ���������Ϊ����Դ��ԭ����ܵ��� ������ĸ����

a������Ȼ���д��ڴ����ĵ��ʹ�
b�������ͨ����ѧ������������
c������н�ǿ�������ԣ�ȼ�շų���������
d����Ļ�ѧ���ʲ����ã����ڰ�ȫ���桢����
II����0.2mol/L HA��Һ��O��lmol/L NaOH��Һ�������ϣ���û����Һc��Na+��>c��A������
�á�>������<����=����д���пհף�
��1�������Һ��c��A����____c��HA����c��HA��+c��A����__0.lmol/L��
��2�������Һ�У���ˮ���������c��OH���� 0.2mol/L HA��Һ����ˮ�������c��H+��
��3��25��ʱ�����ȡ0.2mol/L HB��Һ��0.lmol/L NaOH��Һ�������ϣ���û����Һ��pH <7����HB�ĵ���̶� NaB��ˮ��̶ȡ�
��2����ͼΪ����ˮ��װ�ã�д������ʱ�뵼��缫���淢���ĵ缫��Ӧʽ________��ĿǰӦ�����İ뵼�������Si����������������ǡ�21���͵���Դ���������Ϊ����Դ��ԭ����ܵ��� ������ĸ����

a������Ȼ���д��ڴ����ĵ��ʹ�
b�������ͨ����ѧ������������
c������н�ǿ�������ԣ�ȼ�շų���������
d����Ļ�ѧ���ʲ����ã����ڰ�ȫ���桢����
II����0.2mol/L HA��Һ��O��lmol/L NaOH��Һ�������ϣ���û����Һc��Na+��>c��A������
�á�>������<����=����д���пհף�
��1�������Һ��c��A����____c��HA����c��HA��+c��A����__0.lmol/L��
��2�������Һ�У���ˮ���������c��OH���� 0.2mol/L HA��Һ����ˮ�������c��H+��
��3��25��ʱ�����ȡ0.2mol/L HB��Һ��0.lmol/L NaOH��Һ�������ϣ���û����Һ��pH <7����HB�ĵ���̶� NaB��ˮ��̶ȡ�
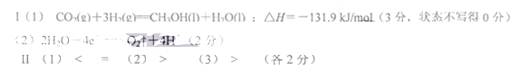
��

��ϰ��ϵ�д�
�����Ŀ
 H++OH�� ��H��0������������ȷ���ǣ� ��
H++OH�� ��H��0������������ȷ���ǣ� ��
 ��CH3COONa��Һ��0.1
��CH3COONa��Һ��0.1