��Ŀ����
������BN����һ����Ҫ�Ĺ����մɲ��ϣ�����Ȼ��ɰΪ��ʼ�����һϵ�з�Ӧ���Եõ�BF3��BN������ͼ��ʾ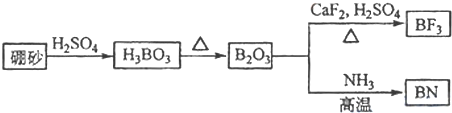
��ش��������⣺
��1����B2O3�Ʊ�BF3��BN�Ļ�ѧ����ʽ������______��______��
��2����̬Bԭ�ӵĵ����Ų�ʽΪ______��B��N��ȣ��縺�Խϴ����______��BN��BԪ�صĻ��ϼ�Ϊ______��
��3����BF3�����У�F-B-F�ļ�����______��Bԭ�ӵ��ӻ��������Ϊ______��BF3����NaF���ÿ�����NaBF4��BF4-������ṹΪ______��
��4������ʯī�ṹ���Ƶ��������������У�����Bԭ����Nԭ��֮��Ļ�ѧ��Ϊ______�����������Ϊ______��
��5�������������ڸ��¸�ѹ�£�����ת��Ϊ������������ṹ����ʯ���ƣ�Ӳ������ʯ�൱�������߳�Ϊ361.5pm�������������к���______����ԭ�ӡ�______����ԭ�ӣ�������������ܶ���______g?pm-3��ֻҪ������ʽ�����ؼ������ֵ������٤������ΪNA����

��ش��������⣺
��1����B2O3�Ʊ�BF3��BN�Ļ�ѧ����ʽ������______��______��
��2����̬Bԭ�ӵĵ����Ų�ʽΪ______��B��N��ȣ��縺�Խϴ����______��BN��BԪ�صĻ��ϼ�Ϊ______��
��3����BF3�����У�F-B-F�ļ�����______��Bԭ�ӵ��ӻ��������Ϊ______��BF3����NaF���ÿ�����NaBF4��BF4-������ṹΪ______��
��4������ʯī�ṹ���Ƶ��������������У�����Bԭ����Nԭ��֮��Ļ�ѧ��Ϊ______�����������Ϊ______��
��5�������������ڸ��¸�ѹ�£�����ת��Ϊ������������ṹ����ʯ���ƣ�Ӳ������ʯ�൱�������߳�Ϊ361.5pm�������������к���______����ԭ�ӡ�______����ԭ�ӣ�������������ܶ���______g?pm-3��ֻҪ������ʽ�����ؼ������ֵ������٤������ΪNA����
��1����ͼ��֪B2O3��CaF2��H2SO4��Ӧ������BF3��ͬʱ��Ӧ�ò�������ƺ�ˮ������ʽΪB2O3+3CaF2+3H2SO4
2BF3��+3CaSO4+3H2O��B2O3�백���ڸ����·�Ӧ������BN������ʽΪ
B2O3+2NH3
2BN+3H2O���ʴ�Ϊ��B2O3+3CaF2+3H2SO4
2BF3��+3CaSO4+3H2O��B2O3+2NH3
2BN+3H2O��
��2����̬Bԭ�ӵĵ����Ų�ʽΪ1s22s2sp1��B��N��λ�ڵڶ����ڣ��縺�Դ����������εݼ�������N�ĵ縺�Դ���B��BN��BԪ�صĻ��ϼ�Ϊ+3���ʴ�Ϊ��1s22s2sp1��N��+3��
��3�����ݼ۲���ӶԻ������ۣ������BF3�ŶԵ��Ӷ���=
����a-xb��=
����4-4��1��=0�����Ҽ۲���Ӷ���Ϊ3������BF3����Ϊƽ���������νṹ������Ϊ120�㣬�ӻ���ʽΪsp2��BF4-����ԭ�ӵŶԵ��Ӷ���=
����a-xb��=
����4-4��1��=0����۲���Ӷ���Ϊ4��������ṹΪ�������壮�ʴ�Ϊ��120�㣻sp2���������壻
��4��B��N�����ڷǽ���Ԫ�أ������γɵĻ�ѧ���Ǽ��Թ��ۼ�������ʯī�ṹ��֪�������������У������֮�俿���Ӽ���������ϣ��ʴ�Ϊ�����ۼ������Թ��ۼ��������Ӽ���������
��5�����ݽ��ʯ�Ľṹ�����жϳ����ʯ��һ�������к��е�̼ԭ����=8��
+6��
+4=8�����һ�������������к���4��Nԭ�Ӻ�4��Bԭ�ӣ�һ�������е�����Ϊ
��4��һ������������������ǣ�361.5pm��3�����������������ܶ���
g?pm-3���ʴ�Ϊ��
��
| ||
B2O3+2NH3
| ||
| ||
| ||
��2����̬Bԭ�ӵĵ����Ų�ʽΪ1s22s2sp1��B��N��λ�ڵڶ����ڣ��縺�Դ����������εݼ�������N�ĵ縺�Դ���B��BN��BԪ�صĻ��ϼ�Ϊ+3���ʴ�Ϊ��1s22s2sp1��N��+3��
��3�����ݼ۲���ӶԻ������ۣ������BF3�ŶԵ��Ӷ���=
| 1 |
| 2 |
| 1 |
| 2 |
| 1 |
| 2 |
| 1 |
| 2 |
��4��B��N�����ڷǽ���Ԫ�أ������γɵĻ�ѧ���Ǽ��Թ��ۼ�������ʯī�ṹ��֪�������������У������֮�俿���Ӽ���������ϣ��ʴ�Ϊ�����ۼ������Թ��ۼ��������Ӽ���������
��5�����ݽ��ʯ�Ľṹ�����жϳ����ʯ��һ�������к��е�̼ԭ����=8��
| 1 |
| 8 |
| 1 |
| 2 |
| 25g |
| NA |
| 25��4 |
| (361.5��10-101��)3NA |
| 25��4 |
| (361.5��10-101��)3NA |

��ϰ��ϵ�д�
�����Ŀ




 ������������������γ���������[ Co(NO2)6]3������������������K�����䷴Ӧ���£�3K����[Co(NO2)6]3��=K3[Co(NO2)6]��(����ɫ)��
������������������γ���������[ Co(NO2)6]3������������������K�����䷴Ӧ���£�3K����[Co(NO2)6]3��=K3[Co(NO2)6]��(����ɫ)��