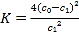��Ŀ����
����Ŀ����ѧ��ȤС���ijƷ��������Ħ�����ɷּ��京����������̽����
������ϣ��ٸ�����Ħ������̼��ơ�����������ɣ�
�������������ɷ���������ʱ���������ɡ�
��.Ħ���������������Ķ��Լ��飺ȡ����������Ʒ����ˮ�ɷֽ��衢���ˡ�
��1���������м������NaOH��Һ�����ˡ�����1��������Һ����ͨ�����������̼���ټ������ϡ���ᡣ�۲쵽��������____________________��
��.������Ʒ��̼��ƵĶ����ⶨ
������ͼ��ʾװ�ã�ͼ�мг�������ȥ������ʵ�飬��ַ�Ӧ�ⶨC�����ɵ�BaCO3������������ȷ��̼��Ƶ�����������

����ʵ����̻ش��������⣺
��2��ʵ����������������ͨ������������ó��˿ɽ���B��C�еķ�Ӧ���⣬����_______��
��3�����и����ʩ�У�������߲ⶨȷ�ȵ���_____________�����ţ���
a.�ڼ�������֮ǰ��Ӧ�ž�װ���ڵ�CO2����
b.�μ�����˹���
c.��A��B֮������ʢ��Ũ�����ϴ��װ��
d.��B��C֮������ʢ�б���̼��������Һ��ϴ��װ��
��4��ʵ����ȷ��ȡ8.00g��Ʒ���ݣ��������βⶨ�����BaCO3ƽ������Ϊ3.94g������Ʒ��̼��Ƶ���������Ϊ_____________________________��
��5��������Ϊ���زⶨC�����ɵ�BaCO3������ֻҪ�ⶨװ��C������CO2ǰ��������һ������ȷ��̼��Ƶ�����������ʵ��֤�����˷����ⶨ�Ľ������ƫ�ߣ�ԭ����__________��
��6�����������AlCl3��NaOH��Һ��ϣ���ַ�Ӧ��û����Һ�к�����Ԫ�������dz����к�����Ԫ��������2������c(AlCl3)��c(NaOH)�ı�ֵ��____________��
���𰸡���1��ͨ��CO2�����а�ɫ�������ɣ������������������ɡ������ܽ⣻
��2�������ɵ�CO2����ȫ������C�У�ʹ֮��ȫ��Ba(OH)2��Һ����
��3��c��d
��4��25%
��5��B�е�ˮ�������Ȼ�������Ƚ���װ��C�� ��6�� 1:1��3:11
��������
���⣨1��ͨ�����CO2ʱ������Ӧ��AlO2����CO2��2H2O=Al(OH)3����HCO3����������ɫ�������ټӹ�������ʱ������Ӧ��Al(OH)3��3H��=Al3����3H2O��HCO3����H��===H2O��CO2������ɫ�����ܽ⣬ͬʱ�������塣��2��װ���в������ֶ�����̼�����ܱ���ȫ���գ����²ⶨ��̼�ᱵ������ƫС����������ͨ�����������Ϊ�������ɵ�CO2����ȫ������C�У�ʹ֮��ȫ��Ba(OH) 2��Һ���գ���3��a���ڼ�������֮ǰ��Ӧ�ž�װ���ڵ�CO2���壬��ֹӰ��̼�ᱵ�����IJⶨ��������߲ⶨȷ�ȣ�a�����ϣ�b���μ�����˹��죬ʹ������̼������ȫ��������߲ⶨȷ�ȣ�b�����ϣ�c����AB֮������ʢ��Ũ�����ϴ��װ�ã�����ˮ�֣���Ӱ��CO2��������߲ⶨȷ�ȣ� c���ϣ�d����BC֮������ʢ�б���̼��������Һ��ϴ��װ�ã��Ȼ����ѱ�Cװ�����գ���Ӱ��CO2��������߲ⶨȷ�ȣ� d���ϣ���4��BaCO3����Ϊ3��94g����n��BaCO3��=![]() =0��02mol����n��CaCO3��=0��02mol������Ϊ0��02mol��100g/mol=2g��������Ʒ��̼��Ƶ���������Ϊ
=0��02mol����n��CaCO3��=0��02mol������Ϊ0��02mol��100g/mol=2g��������Ʒ��̼��Ƶ���������Ϊ![]() ��100%=25%����5��B�е�ˮ�������Ȼ�������Ƚ���װ��C�У����²ⶨ������̼������ƫ�ⶨ��̼��Ƶ�����ƫ��̼��Ƶ���������ƫ�ߣ���6�����ݷ�Ӧ��AlCl3+3NaOH=Al(OH)3��+3HCl��Al(OH)3+ NaOH= NaAlO2+2H2O����Һ����Ԫ�صĴ�����ʽ������Al3����AlO2�����ʵ���Һ��Al3����Al(OH)3���ʵ���֮��Ϊ2��1ʱ��c(AlCl3)��c(NaOH)��3��3=1��1������Һ��AlO2����Al(OH)3�����ʵ���֮��Ϊ2��1ʱ��c(AlCl3)��c(NaOH)��3��11��
��100%=25%����5��B�е�ˮ�������Ȼ�������Ƚ���װ��C�У����²ⶨ������̼������ƫ�ⶨ��̼��Ƶ�����ƫ��̼��Ƶ���������ƫ�ߣ���6�����ݷ�Ӧ��AlCl3+3NaOH=Al(OH)3��+3HCl��Al(OH)3+ NaOH= NaAlO2+2H2O����Һ����Ԫ�صĴ�����ʽ������Al3����AlO2�����ʵ���Һ��Al3����Al(OH)3���ʵ���֮��Ϊ2��1ʱ��c(AlCl3)��c(NaOH)��3��3=1��1������Һ��AlO2����Al(OH)3�����ʵ���֮��Ϊ2��1ʱ��c(AlCl3)��c(NaOH)��3��11��

����Ŀ��ijѧ������֪���ʵ���Ũ�ȵ��������ⶨδ֪���ʵ���Ũ�ȵ�NaOH��Һʱ��ѡ�������ָʾ��������д���пհף�
��1����ͼ��______������A������B�����Ǽ�ʽ�ζ��������и�ʵ��ĵ�һ��������____________________��
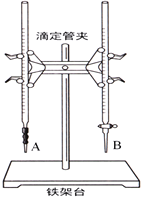
��2���ñ�������ζ������NaOH��Һʱ����������ʽ�ζ��ܵĻ���������ҡ����ƿ���۾�ע����ƿ����Һ��ɫ�仯��ֱ�������һ���������_____________________________��������������˵���ﵽ�ζ��յ㡣
��3�����в����п���ʹ����NaOH��Һ��Ũ����ֵƫ�͵�����____��
A����ʽ�ζ���δ�ñ�������ϴ��ֱ��ע�������
B���ζ�ǰʢ��NaOH��Һ����ƿ������ˮϴ����û�и���
C����ʽ�ζ����ڵζ�ǰ�����ݣ��ζ���������ʧ
D����ȡ�������ʱ����ʼ���Ӷ������ζ�����ʱ���Ӷ���
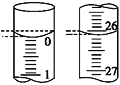
��4�����ζ���ʼ�ͽ���ʱ����ʽ�ζ����е�Һ����ͼ��ʾ��������������Һ�����Ϊ________mL��
��5��ijѧ������3��ʵ��ֱ��¼�й��������±���
�ζ����� ����NaOH��Һ�����/mL | 0.100 0 mol��L��1��������/mL | ||
�ζ�ǰ�̶� | �ζ���̶� | ||
��һ�� | 25.00 | 0.00 | 26.11 |
�ڶ��� | 25.00 | 1.56 | 30.30 |
������ | 25.00 | 0.22 | 26.31 |
�����ϱ�������ʽ������NaOH��Һ�����ʵ���Ũ�ȣ�д����Ҫ���̣���______________