��Ŀ����
���и���Һ�У��йسɷֵ����ʵ���Ũ�ȹ�ϵ��ȷ���ǣ�������
������A���������Һ����Һ�д��ڵ��������Ȼ��ơ���������ᣬ������Ȼ��ⶼ����������ӵ�����Һ�����ԣ����������غ��жϣ�
B��0.1mol/L ��NaHB��ҺpHΪ4��˵��HB-�ĵ���̶ȴ���ˮ��̶ȣ�
C�������Һ�����ԣ���c��OH-��=c��H+������Һ�е������������ơ�����李�һˮ�ϰ���
D���������ӡ�笠�����ˮ�ⶼʹ��Һ�����ԣ���������������笠�����ˮ�⣮
B��0.1mol/L ��NaHB��ҺpHΪ4��˵��HB-�ĵ���̶ȴ���ˮ��̶ȣ�
C�������Һ�����ԣ���c��OH-��=c��H+������Һ�е������������ơ�����李�һˮ�ϰ���
D���������ӡ�笠�����ˮ�ⶼʹ��Һ�����ԣ���������������笠�����ˮ�⣮
����⣺A��n��CH3COONa��=0.5mol/L��0.01L=0.005mol��n��HCl��=1mol/L��0.006L=0.006mol�������������������Ϻ���Һ�е������Ǵ��ᡢ�Ȼ��ơ����ᣬ��������ᶼ����������ӣ�ֻ�д���������������ӣ�����c��H+����c��CH3COO-������A����
B.0.1mol/L ��NaHB��ҺpHΪ4��˵��HB-�ĵ���̶ȴ���ˮ��̶ȣ�����c��B2-����c��H2B������B����
C����Һ�е������������ơ�����李�һˮ�ϰ�����Һ�д��ڵ���غ�c��Na+��+c��NH4+��+c��H+��=2c��SO42-��+c��OH-���������Һ�����ԣ���c��OH-��=c��H+������c��Na+��+c��NH4+��=2c��SO42-���������ӡ���������Ӳ�ˮ�⣬笠�������ˮ�⣬����c��Na+����c��SO42-����c��NH4+������C��ȷ��
D����ͬpH�ģ�NH4��2SO4��NH4Cl��Һ�У�����狀��Ȼ�臨���ǿ�������Σ�������Һ�ʵ����Կ��ж϶���NH4+Ũ����ȣ���2c[��NH4��2SO4]=c��NH4Cl����������������笠�����ˮ�⣬����pH��ȵģ�NH4��2SO4��Һ����NH4��2Fe ��SO4��2��Һ��NH4Cl��Һ��c[��NH4��2SO4]��c��NH4Cl����c[��NH4��2Fe ��SO4��2]����D����
��ѡC��
B.0.1mol/L ��NaHB��ҺpHΪ4��˵��HB-�ĵ���̶ȴ���ˮ��̶ȣ�����c��B2-����c��H2B������B����
C����Һ�е������������ơ�����李�һˮ�ϰ�����Һ�д��ڵ���غ�c��Na+��+c��NH4+��+c��H+��=2c��SO42-��+c��OH-���������Һ�����ԣ���c��OH-��=c��H+������c��Na+��+c��NH4+��=2c��SO42-���������ӡ���������Ӳ�ˮ�⣬笠�������ˮ�⣬����c��Na+����c��SO42-����c��NH4+������C��ȷ��
D����ͬpH�ģ�NH4��2SO4��NH4Cl��Һ�У�����狀��Ȼ�臨���ǿ�������Σ�������Һ�ʵ����Կ��ж϶���NH4+Ũ����ȣ���2c[��NH4��2SO4]=c��NH4Cl����������������笠�����ˮ�⣬����pH��ȵģ�NH4��2SO4��Һ����NH4��2Fe ��SO4��2��Һ��NH4Cl��Һ��c[��NH4��2SO4]��c��NH4Cl����c[��NH4��2Fe ��SO4��2]����D����
��ѡC��
���������⿼��������Ũ�ȴ�С�ıȽϣ���ȷ��Һ�е����ʼ���Һ��������ǽⱾ��ؼ�����ϵ���غ�����������״�ѡ����D��ע���������Ӷ�笠����ӵ�Ӱ�죬Ϊ�״��㣮

��ϰ��ϵ�д�
 �Ͻ�ƽСѧ��������ϵ�д�
�Ͻ�ƽСѧ��������ϵ�д�
�����Ŀ
��10�֣��ڳ����£���һ��Ũ�ȵ�CH3COOH��Һ�ζ�V mLͬŨ��NaOH��Һʱ�õ��ĵζ���������ͼ��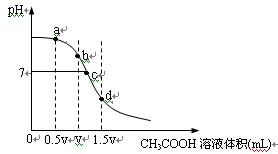
���ⶨij��Һ��ֻ����Na+��CHCOO����H+��OH�� �������ӣ���֪������Һ����һ�ֻ��������ʡ������ϱ�����Ũ�ȵ�CH3COOH��CH3COONa�Ļ��Һ�����ԡ���������и��⣺
��1���Է�����ͼ����ʾ�ζ����̵�b��d������ܵ�������ϣ�
b��_____________________��d��____________________��
��2���ֱ�ָ����ͼa��c���������ڵ���������Ũ�ȴ�С��ϵ��
a�㣺_________________________________________________________________________
c�㣺_________________________________________________________________________
��3��ˮ�ĵ���̶�����Һ�����ܽ�ĵ�����йأ��Է�����ͼa��b��c��d�㣬ˮ�ĵ���̶�������______��
��4���й�������Һ�����е�˵������ȷ����_________
| A������Һ�����Ӽ����㣺c(Na+)>c(CH3COO��)>c(OH��)>c(H+)������Һ�����ʿ���ΪCH3COONa��NaOH |
| B������Һ�����Ӽ�����c(CH3COO��)>c(Na+)>c(H+)>c (OH��)������Һ������һ��ֻ��CH3COONa |
| C������Һ��c(Na+)=c(CH3COO��)�������Һһ�������� |
| D������Һ��c(CH3COOH)>c(Na+)������Һһ�������� |
��5������Һ���������ȵ�CH3COOH��Һ��NaOH��Һ��϶��ɣ���ǡ�ó����ԣ�����ǰc(CH3COOH)____________c(NaOH)���>����<����=������
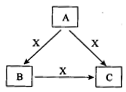
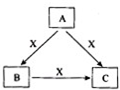 A��B��C��X����ѧ��ѧ�������ʣ����ɶ�����Ԫ����ɣ�ת����ϵ��ͼ��ʾ����������²�ͬ����ش�
A��B��C��X����ѧ��ѧ�������ʣ����ɶ�����Ԫ����ɣ�ת����ϵ��ͼ��ʾ����������²�ͬ����ش�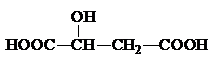 ���÷����й����ŵ�����Ϊ
���÷����й����ŵ�����Ϊ
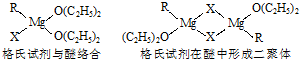
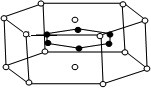
 Ϊ_______________________________________��
Ϊ_______________________________________�� �ṹ��
�ṹ��