��Ŀ����
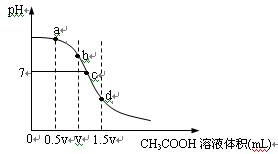
��10�֣��ڳ����£���һ��Ũ�ȵ�CH3COOH��Һ�ζ�V mLͬŨ��NaOH��Һʱ�õ��ĵζ���������ͼ��
���ⶨij��Һ��ֻ����Na+��CHCOO����H+��OH�� �������ӣ���֪������Һ����һ�ֻ��������ʡ������ϱ�����Ũ�ȵ�CH3COOH��CH3COONa�Ļ��Һ�����ԡ���������и��⣺
��1���Է�����ͼ����ʾ�ζ����̵�b��d������ܵ�������ϣ�
b��_____________________��d��____________________��
��2���ֱ�ָ����ͼa��c���������ڵ���������Ũ�ȴ�С��ϵ��
a�㣺_________________________________________________________________________
c�㣺_________________________________________________________________________
��3��ˮ�ĵ���̶�����Һ�����ܽ�ĵ�����йأ��Է�����ͼa��b��c��d�㣬ˮ�ĵ���̶�������______��
��4���й�������Һ�����е�˵������ȷ����_________
| A������Һ�����Ӽ����㣺c(Na+)>c(CH3COO��)>c(OH��)>c(H+)������Һ�����ʿ���ΪCH3COONa��NaOH |
| B������Һ�����Ӽ�����c(CH3COO��)>c(Na+)>c(H+)>c (OH��)������Һ������һ��ֻ��CH3COONa |
| C������Һ��c(Na+)=c(CH3COO��)�������Һһ�������� |
| D������Һ��c(CH3COOH)>c(Na+)������Һһ�������� |
��5������Һ���������ȵ�CH3COOH��Һ��NaOH��Һ��϶��ɣ���ǡ�ó����ԣ�����ǰc(CH3COOH)____________c(NaOH)���>����<����=������
��1��b�㣺CH3COONa(1��)��d�㣺CH3COONa��CH3COOH��1�֣�
��2��a�㣺C��Na+����C(OH_)��C(CH3COO-) ��C(H+)��2�֣�
c�㣺C��Na+��=C(CH3COO-)��C(OH_)=C(H+)����2�֣�
��3��C��1�֣�
��4��B E(2��)
��5������1�֣�
����

�״���һ����Ҫ�Ļ���ԭ�ϡ��״���ˮ�����������ɻ�������Դ�����й㷺��Ӧ��ǰ������������ʵ�飬�����Ϊ1 L���ܱ������У�����1mol CH3OH��1molH2O��һ�������·�����Ӧ��CH3OH (g)+ H2O (g)  CO2(g) +3 H2 (g)�����CO2��CH3OH(g)��Ũ����ʱ��仯���±���ʾ��?
CO2(g) +3 H2 (g)�����CO2��CH3OH(g)��Ũ����ʱ��仯���±���ʾ��?
ʱ�� ���� | 0 min | 10 min | 30 min | 60 min | 70 min |
CO2(mol/L) | 0 | 0.2 | 0.6 | 0.8 | 0.8 |
CH3OH(mol/L) | 1.0 | 0.8 | 0.4 | 0.2 | 0.2 |
����֪��CH3OH (g)+  O2 (g)
O2 (g)  CO2(g) + 2H2 (g)? ?H1= ��192.9kJ/mol?
CO2(g) + 2H2 (g)? ?H1= ��192.9kJ/mol?
H2(g)+  O2 (g)
O2 (g)  H2 O(g)? ?H2= ��120.9kJ/mol?
H2 O(g)? ?H2= ��120.9kJ/mol?
��״���ˮ������������Ӧ���ʱ���H3=_____??????????????????? ��?
��10~30 min�ڣ�������ƽ����Ӧ����v(H2)��___________mol/(L��min)��?
���÷�Ӧ��ƽ�ⳣ������ʽΪK=__________________��?
�����д�ʩ����ʹƽ��ʱn(CH3OH)��n(CO2)��С����(˫ѡ)___________��?
A���������?????????????? B�����ݳ���He(g)��ʹ��ϵѹǿ����?
C����H2(g)����ϵ�з���??? D���ٳ���1molH2O?
��2���״��ڴ��������¿���ֱ�������ɼ��ᡣ?
���ڳ����£���0.1000 mol/L NaOH��Һ�ζ�20. 00 mL 0.1000 mol/L ������Һ�����У������Һ��pH=7ʱ�������ĵ�V(NaOH)___(������������������������) 20. 00 mL��?
���������ζ������У��������ỻ�����ᣬ����ͼ�е���Ӧλ�û�����Ӧ�ĵζ����ߡ�(1����ҺԼ0.04mL)?