��Ŀ����
�л���ѧ֪ʶ��������Ӧ�ù㷺��
��1�����ࡢ��֬�͵������Ƕ����Ժ�ֲ����ʳ���еĻ���Ӫ�����ʣ�
�������й�˵���У���ȷ����
A���ޡ��顢ľ�ġ���˿����Ҫ�ɷֶ�����ά��
B����֬�Dz���������ߵ�Ӫ������
C���������������ڷ���ˮ���������ɰ�����
D����������������
E�����ۡ���ά�ء������ʶ�����Ȼ�߷��ӻ�����
F������炙�����Ǧ��Һ���뵽��������Һ�У������ʶ��ܴ���Һ������
��������������Ҫ����ĵ��ǣ�������һ��Ӫ�����ʣ����������ƾ��ȹ�ҵ�ϣ�д�������Ƿ���������Ӧ�Ļ�ѧ����ʽ��
��2��ƻ���᳣������ˮ���ǹ������Ӽ�����ṹ��ʽΪ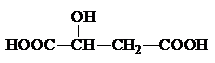 ���÷����й����ŵ�����Ϊ
���÷����й����ŵ�����Ϊ
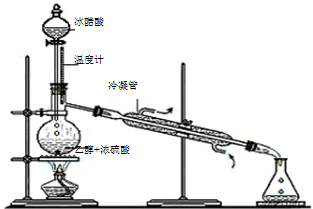
��3��ʵ���Һϳ����������IJ������£���Բ����ƿ�ڼ����Ҵ���Ũ��������ᣬƿ����ֱ��װͨ����ȴˮ�������ܣ�ʹ��Ӧ��������������ΪҺ��������ƿ�ڣ������Ȼ���һ��ʱ�������װ�ý���������ͼ1��ʾ�����õ������Ҵ��������ˮ�����������ֲ�Ʒ����ش��������⣺����֪���Ҵ������ᡢ���������ķе�������78.4�桢118�桢77.1�棩
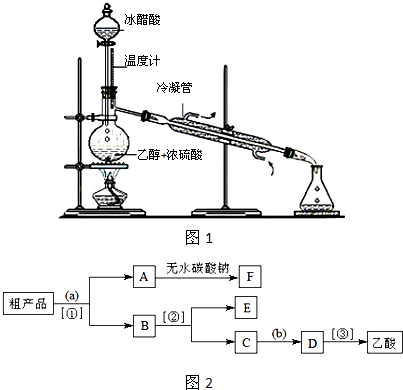
������ƿ�г��˼����Ҵ���Ũ����������⣬��Ӧ���뼸�����Ƭ����Ŀ����
���������������ķ�Ӧ�ǿ��淴Ӧ����Ӧ�ﲻ����ȫ����������Ӧһ��ʱ��ʹﵽ�˸÷�Ӧ���ȣ����ﵽ��ѧƽ��״̬������������˵���÷�Ӧ�Ѵﵽ��ѧƽ��״̬���У�����ţ�
A����λʱ�������1mol����������ͬʱ����1molˮ
B����λʱ�������1mol����������ͬʱ����1mol����
C����λʱ�������1mol�Ҵ���ͬʱ����1mol����
D������Ӧ���������淴Ӧ���������
E��������и����ʵ�Ũ�Ȳ��ٱ仯
��������뺬���ᡢ�Ҵ���ˮ�����������ֲ�Ʒ����ͼ2��ʾ�Ƿ��������������ͼ��
�Լ�a��
��д��C��D ��Ӧ�Ļ�ѧ����ʽ
��1�����ࡢ��֬�͵������Ƕ����Ժ�ֲ����ʳ���еĻ���Ӫ�����ʣ�
�������й�˵���У���ȷ����
BCEF
BCEF
��A���ޡ��顢ľ�ġ���˿����Ҫ�ɷֶ�����ά��
B����֬�Dz���������ߵ�Ӫ������
C���������������ڷ���ˮ���������ɰ�����
D����������������
E�����ۡ���ά�ء������ʶ�����Ȼ�߷��ӻ�����
F������炙�����Ǧ��Һ���뵽��������Һ�У������ʶ��ܴ���Һ������
��������������Ҫ����ĵ��ǣ�������һ��Ӫ�����ʣ����������ƾ��ȹ�ҵ�ϣ�д�������Ƿ���������Ӧ�Ļ�ѧ����ʽ��
CH2OH��CHOH��4CHO+2Ag��NH3��2OH
2Ag��+CH2OH��CHOH��4COONH4+3NH3+H2O
| ˮԡ |
CH2OH��CHOH��4CHO+2Ag��NH3��2OH
2Ag��+CH2OH��CHOH��4COONH4+3NH3+H2O
��| ˮԡ |
��2��ƻ���᳣������ˮ���ǹ������Ӽ�����ṹ��ʽΪ
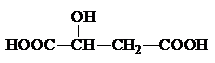 ���÷����й����ŵ�����Ϊ
���÷����й����ŵ�����Ϊ�Ȼ����ǻ�
�Ȼ����ǻ�
�����Ժʹ������ʷ�������
����
��Ӧ�������Է�����������ˮ���������ᣬ��������ʹ��ˮ��ɫ����������Ľṹ��ʽΪHOOC-CH=CH-COOH
HOOC-CH=CH-COOH
����3��ʵ���Һϳ����������IJ������£���Բ����ƿ�ڼ����Ҵ���Ũ��������ᣬƿ����ֱ��װͨ����ȴˮ�������ܣ�ʹ��Ӧ��������������ΪҺ��������ƿ�ڣ������Ȼ���һ��ʱ�������װ�ý���������ͼ1��ʾ�����õ������Ҵ��������ˮ�����������ֲ�Ʒ����ش��������⣺����֪���Ҵ������ᡢ���������ķе�������78.4�桢118�桢77.1�棩

������ƿ�г��˼����Ҵ���Ũ����������⣬��Ӧ���뼸�����Ƭ����Ŀ����
��ֹ��ƿ�е�Һ�屩��
��ֹ��ƿ�е�Һ�屩��
�����������������ķ�Ӧ�ǿ��淴Ӧ����Ӧ�ﲻ����ȫ����������Ӧһ��ʱ��ʹﵽ�˸÷�Ӧ���ȣ����ﵽ��ѧƽ��״̬������������˵���÷�Ӧ�Ѵﵽ��ѧƽ��״̬���У�����ţ�
BDE
BDE
��A����λʱ�������1mol����������ͬʱ����1molˮ
B����λʱ�������1mol����������ͬʱ����1mol����
C����λʱ�������1mol�Ҵ���ͬʱ����1mol����
D������Ӧ���������淴Ӧ���������
E��������и����ʵ�Ũ�Ȳ��ٱ仯
��������뺬���ᡢ�Ҵ���ˮ�����������ֲ�Ʒ����ͼ2��ʾ�Ƿ��������������ͼ��
�Լ�a��
����Na2CO3��Һ
����Na2CO3��Һ
�����뷽��������Һ
��Һ
�����뷽����������
����
���Լ�b����Ũ������
��Ũ������
����д��C��D ��Ӧ�Ļ�ѧ����ʽ
2CH3COONa+H2SO4��2CH3COOH+Na2SO4
2CH3COONa+H2SO4��2CH3COOH+Na2SO4
����������1���ٴ����ʵ���ɺ����ʽǶȷ�������˿���ڵ����ʣ����һ������ζ��������ۺ���ά�������ǣ���û����ζ���������Һ���뵽��������Һ�У���ʹ�����ʷ�������������Ǧ��Һ���뵽��������Һ�У���ʹ�����ʷ������ԣ�
��������Ϊ���ǻ�ȩ�ǻ�ԭ�Ե��ǣ��Դ���д��ѧ����ʽ��
��2�������л���Ľṹ��ʽ���۲캬�еĹ����ţ�����д���ƣ�-COOH��-OH����������Ӧ�����������������ᣬ��������ʹ��ˮ��ɫ��˵������̼̼˫����
��3����Һ�����ʹ�ױ��У�Ӧ�������Ƭ��
�ڻ�ѧ��Ӧ�ﵽƽ��ʱ�����淴Ӧ������ȣ������ʵ�Ũ�Ȳ��䣬�ɴ�������һЩ������Ҳ���䣻
�۴ֲ�Ʒ���������к����������Ҵ����ñ��͵�̼������Һ��Ӧ�����ᣬ�ܽ��Ҵ���ͬʱ���������������ܽ�ȣ�������Һ�ֲ㣬���������ܶȱ�ˮС�������������ϲ㣬Ȼ�����÷�Һ����������������ˮ̼�����������е�ˮ���ɵ�������������Һ�к����Ҵ���̼���ơ������ƣ�������������ռ��Ҵ����������Ҵ�����Һ�м������ᣬ���Եõ����ᣬ�ٽ�����������ռ����
�����������ǿ�����ᣮ
��������Ϊ���ǻ�ȩ�ǻ�ԭ�Ե��ǣ��Դ���д��ѧ����ʽ��
��2�������л���Ľṹ��ʽ���۲캬�еĹ����ţ�����д���ƣ�-COOH��-OH����������Ӧ�����������������ᣬ��������ʹ��ˮ��ɫ��˵������̼̼˫����
��3����Һ�����ʹ�ױ��У�Ӧ�������Ƭ��
�ڻ�ѧ��Ӧ�ﵽƽ��ʱ�����淴Ӧ������ȣ������ʵ�Ũ�Ȳ��䣬�ɴ�������һЩ������Ҳ���䣻
�۴ֲ�Ʒ���������к����������Ҵ����ñ��͵�̼������Һ��Ӧ�����ᣬ�ܽ��Ҵ���ͬʱ���������������ܽ�ȣ�������Һ�ֲ㣬���������ܶȱ�ˮС�������������ϲ㣬Ȼ�����÷�Һ����������������ˮ̼�����������е�ˮ���ɵ�������������Һ�к����Ҵ���̼���ơ������ƣ�������������ռ��Ҵ����������Ҵ�����Һ�м������ᣬ���Եõ����ᣬ�ٽ�����������ռ����
�����������ǿ�����ᣮ
����⣺��1����A���ޡ��顢ľ�ĵ���Ҫ�ɷ�Ϊ��ά�أ�����˿����Ҫ�ɷֵ����ʣ���A����
B������Ӫ�����������ࡢ��֬�������ʵȣ�������֬������������ߣ���B��ȷ��
C���������ǰ���������۲��ˮ���������ɰ����ᣬ��C��ȷ��
D�����һ������ζ��������ۺ���ά�������ǣ���û����ζ����D����
E�����ۡ���ά�ء������ʶ�����Ȼ�߷��ӻ������Է��������ϴ�E��ȷ��
F����������ڷ��ؽ����Σ������������Һ���뵽��������Һ�У���ʹ�����ʷ�������������Ǧ�����ؽ����Σ���������Ǧ��Һ���뵽��������Һ�У���ʹ�����ʷ������ԣ������ʶ��ܴ���Һ����������F��ȷ��
�ʴ�Ϊ��BCEF��
��������Ϊ��ԭ�Ե��ǣ�Ϊ���ǻ�ȩ����������ˮԡ���������·���������Ӧ����Ӧ�ķ���ʽΪCH2OH��CHOH��4CHO+2Ag��NH3��2OH
2Ag��+CH2OH��CHOH��4COONH4+3NH3+H2O��
�ʴ�Ϊ��CH2OH��CHOH��4CHO+2Ag��NH3��2OH
2Ag��+CH2OH��CHOH��4COONH4+3NH3+H2O��
��2�������л���Ľṹ��ʽ��֪���л��ﺬ�к���-COOH��-OH���ֹ����ţ�����Ϊ�Ȼ����ǻ����Ȼ����ǻ����ܷ���������Ӧ��ƻ���ᷢ����������ˮ��Ӧ�ķ���ʽΪHOOCCH��OH��CH2COOH
HOOCCH=CHCOOH+H2O��
�ʴ�Ϊ���ǻ����Ȼ���������HOOC-CH=CH-COOH��
��3����Һ�����ʹ�ױ��У�Ӧ�������Ƭ���ʴ�Ϊ����ֹҺ�����ȱ��У�
����֪���淴ӦCH3CH2OH+CH3COOH
CH3COOCH2CH3+H2O��
A����ѧ��Ӧ����֮�ȵ��ڻ�ѧ������֮�ȣ������Ƿ�ﵽƽ�⣬����1mol����������ͬʱ����1molˮ����A����
B����λʱ�������1mol����������ͬʱ����1mol���ᣬ˵�����淴Ӧ������ȣ���B��ȷ��
C����ѧ��Ӧ����֮�ȵ��ڻ�ѧ������֮�ȣ������Ƿ�ﵽƽ�⣬��λʱ�������1mol�Ҵ���ͬʱ����1mol���ᣬ��C����
D������Ӧ���������淴Ӧ��������ȣ��ﵽƽ��״̬����D��ȷ��
E��������и����ʵ�Ũ�Ȳ��ٱ仯��˵���ﵽƽ��״̬����E��ȷ��
�ʴ�Ϊ��BDE��
�۴ֲ�Ʒ���������к����������Ҵ����ñ��͵�̼������Һ��Ӧ�����ᣬ�ܽ��Ҵ���ͬʱ���������������ܽ�ȣ�������Һ�ֲ㣬���������ܶȱ�ˮС�������������ϲ㣬Ȼ�����÷�Һ����������������ˮ̼�����������е�ˮ���ɵ�������������Һ�к����Ҵ���̼���ơ������ƣ�������������ռ��Ҵ����������Ҵ�����Һ�м������ᣬ���Եõ����ᣬ�ٽ�����������ռ����
�ʴ�Ϊ������Na2CO3��Һ����Һ������Ũ�����
��������Ϊ�����Σ�����Ϊǿ�ᣬ����ǿ�������ᣬ2CH3COONa+H2SO4��2CH3COOH+Na2SO4���ʴ�Ϊ��2CH3COONa+H2SO4��2CH3COOH+Na2SO4��
B������Ӫ�����������ࡢ��֬�������ʵȣ�������֬������������ߣ���B��ȷ��
C���������ǰ���������۲��ˮ���������ɰ����ᣬ��C��ȷ��
D�����һ������ζ��������ۺ���ά�������ǣ���û����ζ����D����
E�����ۡ���ά�ء������ʶ�����Ȼ�߷��ӻ������Է��������ϴ�E��ȷ��
F����������ڷ��ؽ����Σ������������Һ���뵽��������Һ�У���ʹ�����ʷ�������������Ǧ�����ؽ����Σ���������Ǧ��Һ���뵽��������Һ�У���ʹ�����ʷ������ԣ������ʶ��ܴ���Һ����������F��ȷ��
�ʴ�Ϊ��BCEF��
��������Ϊ��ԭ�Ե��ǣ�Ϊ���ǻ�ȩ����������ˮԡ���������·���������Ӧ����Ӧ�ķ���ʽΪCH2OH��CHOH��4CHO+2Ag��NH3��2OH
| ˮԡ |
�ʴ�Ϊ��CH2OH��CHOH��4CHO+2Ag��NH3��2OH
| ˮԡ |
��2�������л���Ľṹ��ʽ��֪���л��ﺬ�к���-COOH��-OH���ֹ����ţ�����Ϊ�Ȼ����ǻ����Ȼ����ǻ����ܷ���������Ӧ��ƻ���ᷢ����������ˮ��Ӧ�ķ���ʽΪHOOCCH��OH��CH2COOH
| Ũ���� |
| �� |
�ʴ�Ϊ���ǻ����Ȼ���������HOOC-CH=CH-COOH��
��3����Һ�����ʹ�ױ��У�Ӧ�������Ƭ���ʴ�Ϊ����ֹҺ�����ȱ��У�
����֪���淴ӦCH3CH2OH+CH3COOH
| Ũ���� |
| ���� |
A����ѧ��Ӧ����֮�ȵ��ڻ�ѧ������֮�ȣ������Ƿ�ﵽƽ�⣬����1mol����������ͬʱ����1molˮ����A����
B����λʱ�������1mol����������ͬʱ����1mol���ᣬ˵�����淴Ӧ������ȣ���B��ȷ��
C����ѧ��Ӧ����֮�ȵ��ڻ�ѧ������֮�ȣ������Ƿ�ﵽƽ�⣬��λʱ�������1mol�Ҵ���ͬʱ����1mol���ᣬ��C����
D������Ӧ���������淴Ӧ��������ȣ��ﵽƽ��״̬����D��ȷ��
E��������и����ʵ�Ũ�Ȳ��ٱ仯��˵���ﵽƽ��״̬����E��ȷ��
�ʴ�Ϊ��BDE��
�۴ֲ�Ʒ���������к����������Ҵ����ñ��͵�̼������Һ��Ӧ�����ᣬ�ܽ��Ҵ���ͬʱ���������������ܽ�ȣ�������Һ�ֲ㣬���������ܶȱ�ˮС�������������ϲ㣬Ȼ�����÷�Һ����������������ˮ̼�����������е�ˮ���ɵ�������������Һ�к����Ҵ���̼���ơ������ƣ�������������ռ��Ҵ����������Ҵ�����Һ�м������ᣬ���Եõ����ᣬ�ٽ�����������ռ����
�ʴ�Ϊ������Na2CO3��Һ����Һ������Ũ�����
��������Ϊ�����Σ�����Ϊǿ�ᣬ����ǿ�������ᣬ2CH3COONa+H2SO4��2CH3COOH+Na2SO4���ʴ�Ϊ��2CH3COONa+H2SO4��2CH3COOH+Na2SO4��
���������⿼���Ϊ�ۺϣ��漰�л������ɡ��ṹ�������Լ������������Ʊ���ע������������Ӧ�Ŀ������ص㣬ע���ж�ƽ��״̬�ķ�����Ϊ�״��㣬��Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
�����Ŀ
�л���ѧ֪ʶ��������Ӧ�ù㷺������˵������ȷ���ǣ�������
| A�������Ҵ��ܹ��ܽ�ܶ��л����������Կ����Ҵ���ȡ��ҩ�е�ijЩ�ɷ� | B��������Ȼ����ȼ�Ͽ�����Ч�ؼ��١�����ЧӦ����������� | C�������յķ������Լ���ë֯�����֯�� | D����֬�Dz���������ߵ�Ӫ�����ʣ�����ʳ�б��������֬�������� |
 �л���ѧ֪ʶ��������Ӧ�ù㷺��
�л���ѧ֪ʶ��������Ӧ�ù㷺��