��Ŀ����
��1��ij��Һ���ԼΪ6mL��Ӧѡ����2������50g 25%��NaCl��ҺӦѡ�õ���������Ʒ�У�������ƽ�������룩��ҩ�ס�����ֽ��������
��3������500mL 2mol?L-1��NaOH��Һ��
��Ӧ��������ƽ��
�����ƹ������õ��IJ����������ձ�����Ͳ�������⣬����Ҫ
����������ᵼ������NaOH��ҺŨ�ȴ���0.2mol?L-1����
A��������մ������ B��û����ȴ�����¾Ͷ���
C������ʱ���ӿ̶��� D��������Һ����ڿ̶���
E������ƿϴ�������������ˮ F������ʱ����ˮ�Ӷ����ý�ͷ�ι�������
��������1����ͲѡȡҪѡ������ȡ���������ӽ��ģ���Ͳ�Ķ�����������Һ�尼Һ�����ʹ�����ˮƽ��
��2������25%���Ȼ�����Һ��Ҫȡһ���Ȼ����ܽ���һ����ˮ�У�ʵ�鲽��Ϊ����-����-�ܽ⣻
��3�������������и�ʴ�ԣ��׳��⣻����m=CVM�������ʵ�������
�ڸ���ʵ��������輰������������ѡȡ������
�۸���c=
�����ж������nƫ���VƫС��������ҺŨ��ƫ�ߣ�
��2������25%���Ȼ�����Һ��Ҫȡһ���Ȼ����ܽ���һ����ˮ�У�ʵ�鲽��Ϊ����-����-�ܽ⣻
��3�������������и�ʴ�ԣ��׳��⣻����m=CVM�������ʵ�������
�ڸ���ʵ��������輰������������ѡȡ������
�۸���c=
| n |
| V |
����⣺��1����ͲѡȡҪѡ������ȡ���������ӽ��ģ�������ȡ6mLˮ��Ӧѡ��10mL��Ͳ����Ͳ�Ķ�����������Һ�尼Һ�����ʹ�����ˮƽ����������Ͳ�����������Ķ���ƫ����ȡ��ʵ��Һ�����ƫС���ʴ�Ϊ��10������
��2�������Ȼ�����ʹ��������ƽ���г�ȡ���ܼ�ˮ��Ҫʹ����Ͳ��ȡ���ܽ������ձ����ò��������н����Լӿ��ܽ⣬�ʴ�Ϊ����Ͳ���ձ���
��3�������������и�ʴ�ԣ��׳��⣬Ӧ��С�ձ��г�����m=CVM=2mol/L��0.5L��40g/mol=40g���ʴ�Ϊ��С�ձ���40��
��ʵ������IJ��裺���㡢�������ܽ⡢��Һ��ϴ����Һ�����ݡ�ҡ�ȵȲ�������ʵ������Ҫ����ƽ��������ҩ��ȡҩƷ���ձ��ܽ�ҩƷ����Ҫ�������������������Ҫ500mL����ƿ������Һ����Ҫ��ͷ�ιܶ��ݣ��ʴ�Ϊ��500mL����ƿ����ͷ�ιܣ�
��A��������մ�����ۣ���ȡ��������������ƫ��Ũ��ƫ��A��ȷ��
B��û����ȴ�����¾Ͷ��ݣ���Һ���ƫС��Ũ��ƫ��B��ȷ��
C������ʱ���ӿ̶��ߣ���Һ���ƫС��Ũ��ƫ��C��ȷ��
D��������Һ����ڿ̶�����Ӱ�죬ԭ����Һ��ճ�������ϣ���D����
E������ƿ��������ˮϴ����û�к�ɣ���Ӱ�죬Ũ�Ȳ��䣬��E����
F������ʱ����С�ļ�ˮʹҺ����ڿ��ߺ�����������ˮʹ��Һ����̶������У����ʵ��������٣�Ũ��ƫС����F����
�ʴ�Ϊ��AB��
��2�������Ȼ�����ʹ��������ƽ���г�ȡ���ܼ�ˮ��Ҫʹ����Ͳ��ȡ���ܽ������ձ����ò��������н����Լӿ��ܽ⣬�ʴ�Ϊ����Ͳ���ձ���
��3�������������и�ʴ�ԣ��׳��⣬Ӧ��С�ձ��г�����m=CVM=2mol/L��0.5L��40g/mol=40g���ʴ�Ϊ��С�ձ���40��
��ʵ������IJ��裺���㡢�������ܽ⡢��Һ��ϴ����Һ�����ݡ�ҡ�ȵȲ�������ʵ������Ҫ����ƽ��������ҩ��ȡҩƷ���ձ��ܽ�ҩƷ����Ҫ�������������������Ҫ500mL����ƿ������Һ����Ҫ��ͷ�ιܶ��ݣ��ʴ�Ϊ��500mL����ƿ����ͷ�ιܣ�
��A��������մ�����ۣ���ȡ��������������ƫ��Ũ��ƫ��A��ȷ��
B��û����ȴ�����¾Ͷ��ݣ���Һ���ƫС��Ũ��ƫ��B��ȷ��
C������ʱ���ӿ̶��ߣ���Һ���ƫС��Ũ��ƫ��C��ȷ��
D��������Һ����ڿ̶�����Ӱ�죬ԭ����Һ��ճ�������ϣ���D����
E������ƿ��������ˮϴ����û�к�ɣ���Ӱ�죬Ũ�Ȳ��䣬��E����
F������ʱ����С�ļ�ˮʹҺ����ڿ��ߺ�����������ˮʹ��Һ����̶������У����ʵ��������٣�Ũ��ƫС����F����
�ʴ�Ϊ��AB��
���������⿼����һ�����ʵ���Ũ����Һ�������Լ����������ѶȲ���ע��ʵ��Ļ�������������ע������������ʵ�����������ƿ�Ĺ�������㣮

��ϰ��ϵ�д�
�����Ŀ
ij̽��С�������ӡˢ��·�壨��Cu��Al����Au��Pt�Ƚ����Ļ�������Cu���Ʊ�����������[Al2��SO4��3��18H2O]�����·�����£�
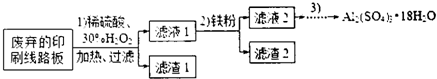
��1������ʱ����Ҫ�IJ��������в������� �� ��
��2��ʵ��ʱ�������2����ϴ�ӣ��ж�ϴ���Ƿ�ɾ���ʵ����������� ��
��3��Ϊȷ���������۵�����ʵ������ⶨ��Һ1��Cu2+������ʵ�����Ϊ����ȥH2O2��ȷ��ȡһ�������Һ1�ڴ�����ƿ�У���ˮϡ�ͣ�������ҺpH=3-4���������KI-������Һ����Na2S2O3����Һ�ζ����յ㣮���������з�Ӧ�����ӷ���ʽ��2Cu2++4I-=2CuI����ɫ����+I2��2S2O32-=2I-+S4O62-
�ٵζ�����ע��Na2S2O3����Һ֮ǰ��Ҫ��������ˮϴ�������� ��
�ڵζ��յ�۲쵽������Ϊ ��
�����ζ�ǰ��Һ�е�H2O2û�г��������ⶨ��Cu2+�������� ���ƫ�ߡ�����ƫ�͡����䡱��
��4������Һ2��ȡ���������壬̽��С����������¶��ַ�����
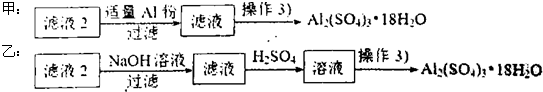
�ٲ����۵�ʵ�鲽������Ϊ������Ũ���� �� ��ϴ�ӣ�
�ڴ�ԭ�������ʽǶȿ��ǣ� ����������������ס����ҡ���
��5�����Dz��������ϣ���Ϊͨ�����������ٵ�����ҺpHҲ�ɽ���Һ2�е�Fe2+��ȥ���±��г��˼������������������������pH����ʼ������pH����������Ũ��Ϊ1.0mol?L-1���㣩��
�����������H2O2������Cl2��ԭ���� ��
�ڵ�����ҺpHԼΪ ��

��1������ʱ����Ҫ�IJ��������в�������
��2��ʵ��ʱ�������2����ϴ�ӣ��ж�ϴ���Ƿ�ɾ���ʵ�����������
��3��Ϊȷ���������۵�����ʵ������ⶨ��Һ1��Cu2+������ʵ�����Ϊ����ȥH2O2��ȷ��ȡһ�������Һ1�ڴ�����ƿ�У���ˮϡ�ͣ�������ҺpH=3-4���������KI-������Һ����Na2S2O3����Һ�ζ����յ㣮���������з�Ӧ�����ӷ���ʽ��2Cu2++4I-=2CuI����ɫ����+I2��2S2O32-=2I-+S4O62-
�ٵζ�����ע��Na2S2O3����Һ֮ǰ��Ҫ��������ˮϴ��������
�ڵζ��յ�۲쵽������Ϊ
�����ζ�ǰ��Һ�е�H2O2û�г��������ⶨ��Cu2+��������
��4������Һ2��ȡ���������壬̽��С����������¶��ַ�����

�ٲ����۵�ʵ�鲽������Ϊ������Ũ����
�ڴ�ԭ�������ʽǶȿ��ǣ�
��5�����Dz��������ϣ���Ϊͨ�����������ٵ�����ҺpHҲ�ɽ���Һ2�е�Fe2+��ȥ���±��г��˼������������������������pH����ʼ������pH����������Ũ��Ϊ1.0mol?L-1���㣩��
| ��ʼ������pH | ������ȫ��pH | |
| Fe3+ | 1.1 | 3.2 |
| Fe2+ | 5.8 | 8.8 |
| Al3+ | 3.8 | 5.2 |
�ڵ�����ҺpHԼΪ
 ij������ȤС�����ⶨijNaOH��Һ��Ũ�ȣ�������������£�
ij������ȤС�����ⶨijNaOH��Һ��Ũ�ȣ�������������£�