��Ŀ����
����Ŀ��Cu2O��Ϳ�ϡ���ɫ�����ʹ������������Ź㷺����;��Cu2OΪ��ɫ��ĩ��������ˮ�������������ϡ���ᡣ���õ�ⷨ�Ʊ�������ͭ����ͭ����������Ƭ�����������ҺΪһ��Ũ�ȵ�NaCl��NaOH�Ļ����Һ������������Һ���й�ת����ͼ��ʾ������˵���������
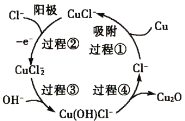
A.���Һ�е�NaOH�������������
B.���̢���Cu����������CuCl��
C.���̢ܵ����ӷ���ʽΪ2Cu(OH)Cl��=Cu2O+2Cl��+H2O
D.����·����0.05mol e��ͨ��ʱ������0.32g Cu
���𰸡�BD
��������
A.����Cu2O�����������ϡ���ᣬ��NaOH������������棬A��ȷ��
B.��ͼʾ��֪�����̢���Ϊ�������̣�ͭԪ�ػ��ϼ�δ�䣬δ����������ԭ��Ӧ��B����
C.����ͼʾ���̢���Cu(OH)Cl����Ӧ����Cu2O��Cl���������ӷ���ʽΪ2Cu(OH)Cl��=Cu2O+2Cl��+H2O��C��ȷ��
D.���������е���ͭ��Ӧ������Cu2O��ͭԪ�ػ��ϼ���0�۱�Ϊ+1�ۣ��ʵ���·����0.05mol e��ͨ��ʱ������Cu������Ϊ64g/mol![]() 0.05mol=3.2g��D����
0.05mol=3.2g��D����
��ѡBD��

����Ŀ��ΪӦ��ʯ�Ͷ�ȱ��һ̼��ѧ�о����ܹ�ע��һ̼��ѧ��ָ�Է�����ֻ��һ��̼ԭ�ӵĻ�������״���һ����̼��Ϊԭ�ϣ������Ʒ�Ļ�ѧ��ϵ���ܳơ�
(1)CH3OH(g)��NH3(g)��һ�������·�Ӧ���Ƶüװ�CH3NH2(g)��
![]()
![]()
����֪�÷�Ӧ����صĻ�ѧ�������������£�
���ۼ� | C��O | N��H | C��N | C��H |
E��(kJ��mol) | a | b | c | d |
��H��O���ļ���Ϊ_________________kJ��mol(�ú�����ĸ�Ĵ���ʽ��ʾ)
����ij�����ܱ������н��и÷�Ӧ�������������������£��ֱ�����ʼʱCH3OH(g)�����ʵ������¶ȶ�ƽ��ʱCH3NH2(g)�����������Ӱ�죬��ͼ��ʾ��(ͼ��T1��T2��ʾ�¶�)
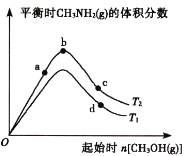
��T1_________T2(���������������=��)��____________(�a������b����c��)���Ӧ��ƽ��״̬�з�Ӧ��NH3(g)��ת�������b��d�����ƽ�ⳣ����С��ϵΪKb________Kd(���������������=��)��
(2)�״��ڹ�ҵ�Ͽ�����ˮú�����ϳɣ�![]() ����1mol CO��2mol H2ͨ���ܱ������н��з�Ӧ����һ���¶Ⱥ�ѹǿ�´ﵽƽ��ʱ�״����������
����1mol CO��2mol H2ͨ���ܱ������н��з�Ӧ����һ���¶Ⱥ�ѹǿ�´ﵽƽ��ʱ�״����������![]() (CH3OH)�仯������ͼ��ʾ��
(CH3OH)�仯������ͼ��ʾ��
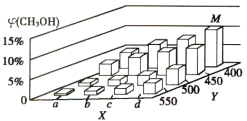
ͼ��Y���ʾ���������Ϊ________________���жϵ�������______________________��
��֪v(��)=k(��)��p(CO)��p(H2)2��v(��)=k(��)��p(CH3OH)������k(��)��k(��)�ֱ�Ϊ�����淴Ӧ���ʳ�����pΪ����ֵķ�ѹ����M���������¶�(T3��)��ѹǿ(p0kPa)�£���Ӧ��20���Ӵﵽƽ��ʱ![]() (CH3OH)=10�������¶��·�Ӧ��ƽ����KP=____________kPa��2(��ƽ���ѹ����ƽ��Ũ�ȼ��㣬��ѹ=��ѹ�����ʵ�������)������15����ʱ
(CH3OH)=10�������¶��·�Ӧ��ƽ����KP=____________kPa��2(��ƽ���ѹ����ƽ��Ũ�ȼ��㣬��ѹ=��ѹ�����ʵ�������)������15����ʱ![]() ����ʱ
����ʱ![]() ______________(������������λС��)
______________(������������λС��)