��Ŀ����
����Ŀ��������G��һ��ҽҩ�м��壬�������Ʊ�����Ѫҩ������ͨ����ͼ��ʾ��·�ߺϳ�

��֪��RCOOH ![]() RCOCl��D��FeCl3��Һ�ܷ�����ɫ��
RCOCl��D��FeCl3��Һ�ܷ�����ɫ��
(1)A��B�ķ�Ӧ������___________��B��C��ת�����ӵ��Լ��ٿ�����______��
(2)E�Ľṹ��ʽΪ_________��
(3)F������NaOH��Һ��ַ�Ӧ�Ļ�ѧ����ʽΪ_________________��
(4)д��ͬʱ������������������E��ͬ���칹��Ľṹ��ʽ____________��
���ܷ���ˮ�ⷴӦ����FeCl3��Һ�ܷ�����ɫ��Ӧ�۱����������ֲ�ͬ��ѧ��������ԭ��
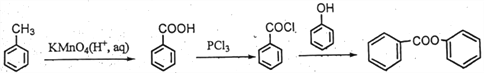
(5)��֪�����ǻ�һ�㲻��ֱ���������������������ᱽ������![]() ����һ����Ҫ���л��ϳ��м��塣��д���Ա��ӡ��ױ�Ϊԭ����ȡ�û�����ĺϳ�·������ͼ(��ԭ������)��
����һ����Ҫ���л��ϳ��м��塣��д���Ա��ӡ��ױ�Ϊԭ����ȡ�û�����ĺϳ�·������ͼ(��ԭ������)��
ע���ϳ�·�ߵ���д��ʽ��������ʾ������ͼ��________________________________

���𰸡� ������Ӧ ���Ƶ�������Һ�����Ƶ�������ͭ����Һ ![]()
![]() +3NaOH
+3NaOH![]()
![]() +CH3COONa+H2O+CH3OH
+CH3COONa+H2O+CH3OH 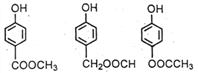
����������F��֪EΪ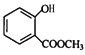 ������D�ķ���ʽ��֪DΪ
������D�ķ���ʽ��֪DΪ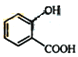 ���ɿ�֪CΪCH3COOH��BΪCH3CHO��AΪCH3CH2OH��(1) A��B��CH3CH2OH������Ӧ����CH3CHO����Ӧ������������Ӧ��B��C��ת������ȩ�������ữת��Ϊ���ᣬ���ӵ��Լ��ٿ��������Ƶ�������Һ�����Ƶ�������ͭ����Һ��(2)E�Ľṹ��ʽΪ
���ɿ�֪CΪCH3COOH��BΪCH3CHO��AΪCH3CH2OH��(1) A��B��CH3CH2OH������Ӧ����CH3CHO����Ӧ������������Ӧ��B��C��ת������ȩ�������ữת��Ϊ���ᣬ���ӵ��Լ��ٿ��������Ƶ�������Һ�����Ƶ�������ͭ����Һ��(2)E�Ľṹ��ʽΪ ��(3) F��
��(3) F��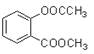 ��������NaOH��Һ��ַ�Ӧ�Ļ�ѧ����ʽΪ
��������NaOH��Һ��ַ�Ӧ�Ļ�ѧ����ʽΪ![]() +3NaOH
+3NaOH![]()
![]() +CH3COONa+H2O+CH3OH��(4) ����E��
+CH3COONa+H2O+CH3OH��(4) ����E�� ����ͬ���칹���ܷ���ˮ�ⷴӦ��˵������������FeCl3��Һ�ܷ�����ɫ��Ӧ��˵���з��ǻ��������������ֲ�ͬ��ѧ��������ԭ�ӣ�˵���Ƕ�λ�ṹ�����Եõ�
����ͬ���칹���ܷ���ˮ�ⷴӦ��˵������������FeCl3��Һ�ܷ�����ɫ��Ӧ��˵���з��ǻ��������������ֲ�ͬ��ѧ��������ԭ�ӣ�˵���Ƕ�λ�ṹ�����Եõ�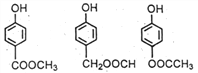 ��(5) �ױ��������ɱ����ᣬ��ȡ�������ȱ���ˮ�����ɱ��ӣ��Դ˺ϳɸ��л���ϳ�����ͼΪ��
��(5) �ױ��������ɱ����ᣬ��ȡ�������ȱ���ˮ�����ɱ��ӣ��Դ˺ϳɸ��л���ϳ�����ͼΪ��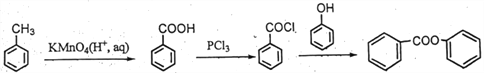 ��
��






