��Ŀ����
����Ŀ����4���ǻ��㶹�أ��Ǻϳ�ҩ�����Ҫ�м��壬�������ϳ�;�����£�

��֪����F�Ľṹ��ʽΪ ��
��
�� ( X=O��N��S��R����)��
( X=O��N��S��R����)��
��RCOOR+2R��OH��R��COOR��+2ROH
�ص��������⣺
(1)����4���ǻ��㶹�ص�˵����ȷ����____________��(����ĸ)
a.��ʹ��ˮ��ɫ b.1mol �������������4mol NaOH��Ӧ
c.�����������9��̼ԭ�ӹ�ƽ�� d.���ڱ���ͬϵ��
(2)A����B�ķ�Ӧ����Ϊ__________��D�Ľṹ��ʽΪ__________��
(3)I������Ϊ_______________��
(4)д��G����H�Ļ�ѧ����ʽ________________________��
(5)������������������F���ṹ��ʽ����֪����ͬ���칹�����Ŀ��__________�֣�
a.�ܷ���������Ӧ b.���ڱ��Ķ�ȡ���� c.ֻ����һ����
д������ͬ���칹������ʹFeCl3��Һ����ɫ���˴Ź���������������������Ϊ1:2:2:2:1�Ľṹ��ʽ______________��
(6)�������֪ʶ����Ϣ�����������ϳ�·�ߣ��Ա���Ϊ��ʼԭ��(���Լ���ѡ)����Ʊ� �ĺϳ�·��______________��
�ĺϳ�·��______________��
���𰸡� ac ������Ӧ ![]() ���������(1��3-������)
���������(1��3-������) 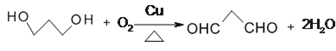 12
12 ![]()
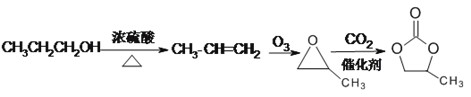
�������������������1������4���ǻ��㶹�صĽṹ��ʽ���ж���
(2)��ϩ���ɻ��������������������ԭ�ӣ�����RCOOR+2R��OH��R��COOR��+2ROH�����E�Ľṹ��ʽ�ƶ�D��
(3) ![]() �����Ȼ����������������ԭ��������
�����Ȼ����������������ԭ��������
(4) ![]() ������Ϊ
������Ϊ![]() ��
��
(5) a.�ܷ���������Ӧ˵������ȩ����������� b.��������2��ȡ���� c.ֻ����һ��������������дͬ���칹����
(6)�Ա�����ȥ���ɱ�ϩ����ϩ���ó�������Ϊ�����������̼��Ӧ����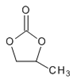 ��
��
��������1������4���ǻ��㶹�صĽṹ��ʽ������̼̼˫������ʹ��ˮ��ɫ��a��ȷ�� 1mol �������������2mol NaOH��Ӧ����b�����������������9��̼ԭ�ӹ�ƽ������c��ȷ�� d.����������̼̼˫���������ڱ���ͬϵ������d������
(2)��ϩ���ɻ��������������������ԭ������������������Ӧ��
����RCOOR+2R��OH��R��COOR��+2ROH�����E�Ľṹ��ʽ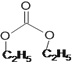 �ƶ�D��
�ƶ�D��![]() ��
��
(3) ![]() �����Ȼ����������������ԭ��������
�����Ȼ����������������ԭ��������![]() �������DZ����ᡣ
�������DZ����ᡣ
(4) ![]() ������Ϊ
������Ϊ![]() �ķ���ʽ��
�ķ���ʽ��![]() ��
��
(5) a.�ܷ���������Ӧ˵������ȩ����������� b.��������2��ȡ���� c.ֻ����һ���������ϸ�������������F��ͬ���칹����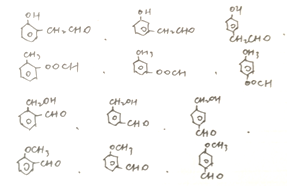
��12����������ʹFeCl3��Һ����ɫ���˴Ź���������������������Ϊ1:2:2:2:1�Ľṹ��ʽ��![]() ��
��
(6)�Ա�����ȥ���ɱ�ϩ����ϩ���ó�������Ϊ![]() ��
��![]() ���������̼��Ӧ����
���������̼��Ӧ���� ������ͼ��
������ͼ��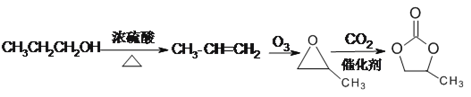

����Ŀ�����б���������ȷ�����������ϵ����(����)
ѡ�� | ������ | ������ |
A | ����������Ũ�����з����ۻ� | �������۳��ܷ�����Ũ���� |
B | SO2��Ư���� | SO2ͨ��Ʒ����Һ������Һ��ɫ |
C | ���ȶ��ԣ�Na2CO3��NaHCO3 | ͬŨ����Һ���ԣ�Na3CO3��NaHCO3 |
D | �ǽ����ԣ�F��Cl | �⻯����ȶ��ԣ�HF��HCl |
A. A B. B C. C D. D




