��Ŀ����
��ѧ��ȤС���ͬѧΪ�ⶨijNa2CO3��NaCl�Ĺ���������Ʒ��Na2CO3��������������������ʵ�飬������벢��ɶ��й�����Ľ��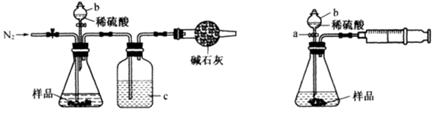
ͼ1 ͼ2
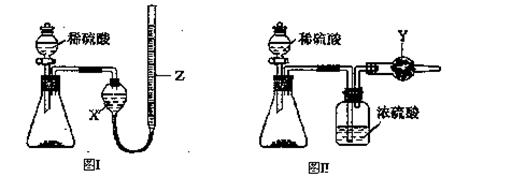
��1����ͬѧ��ͼ1��ʾװ�òⶨCO2��������ʵ��ʱϡ����������Ʒ�е� (�Na2CO3����"NaCl��)������Ӧ������b�������� ��ϴ��ƿc��ʢװ����Ũ���ᣬ��Ũ����������� ��
��2����ͬѧ��ͼ2��ʾװ�ã�ȡһ����������Ʒ(Ϊm g���Ѳ��)������ϡ���ᷴӦ����ʵ�飬�����Ʒ��Na2CO3�����������IJⶨ��
��ʵ��ǰ������װ�������Եķ������ȴ���a����bע��ˮ�����¶˲��������γ�һ��ˮ�����ٽ���Ͳ����������ѹ����b�¶˲������е� ��������װ�����������á�
����ʵ�����ʱ����ֱ�Ӳ�õ�������CO2�� (������������������)��
��3����ͬѧ����ͼ��ʾ�����Ͳ���ʵ�飺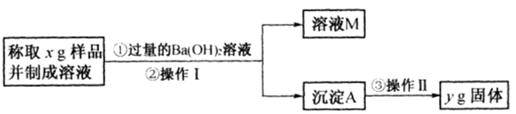
�ٲ���I�漰��ʵ�������� ��ϴ�ӣ��������漰��ʵ�������и�� ��
�ڱ���õ���Ʒ��Na2CO3���������ļ���ʽΪ ��
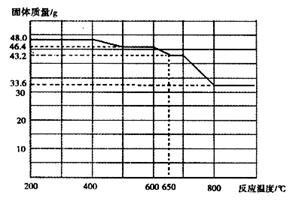
��4����״���£���672 mL CO2��ͨ��50 mL1mol/LKOH��Һ�У���ȫ��Ӧ��������Һ��K2CO3��KHCO3�����ʵ���֮��Ϊ(�跴Ӧǰ����Һ����仯���Բ���) ��
��1��Na2CO3 (1��) ��Һ©��(1��) ��ȥCO2�е�ˮ����(1��)
��2����Һ��(1��)�����(1��)
��3���ٹ���(1��) ����(1��) ��106y/197x (1��)
��4��n(K2CO3):n(KHCO3)=2:1(2��)
���������������1�� NaCl����ϡ���ᷴӦ����ѡNa2CO3������b�������Ƿ�Һ©����Ũ����������dz�ȥCO2�е�ˮ�����������CO2���壩����2�� �ٽ���Ͳ����������ѹ�������������е�ѹǿ������b�¶˲������е�Һ����������װ�����������á���CO2�����壬����ֱ�Ӳ�õ�������CO2���������3�� �ٳ������ɣ��ʲ���I��Ҫ�漰���˲�����Ҫ֪�������������Ҫ���ء��ھ�����ѧʽ�ļ��㣬��Ʒ��Na2CO3���������ļ���ʽΪ106y/197x����4�������ķ�ӦΪ0.03CO2+0.05KOH=XK2CO3+YKHCO3+0.025H2O��X+Y=0.03��2X+Y=0.05�����X=0.02��Y=0.01������Һ��K2CO3��KHCO3�����ʵ���֮��Ϊn(K2CO3):n(KHCO3)0.02��0.01=2:1��
���㣺���⿼���ȥijNaCl��Ʒ�е�����Na2CO3����Ʒ��Na2CO3�������������漰����ԭ������ѧ̽�����̣���һ���ۺ���ǿ�ĺ��⡣

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�������������(FeC2O4��2H2O)���������Լ�����Ӱ�������͵�ز�����������﮵��������ش��������⣺
I����ȤС��Բ�����������ķֽ�������ʵ���̽����̽���ֽ�õ��Ĺ����������Ԫ�صĴ�����ʽ��
��1���������
����һ��___________�� �������ȫ����FeO �� ��������FeO��Fe����
��2�����ʵ�鷽��֤����������
| ʵ�鲽�� | ��������� |
| ����1�����Թ��м��������������ټ������� ������� | ����Һ��ɫ���Ըı࣬���� ���ɣ���֤���������ʴ��� |
| ����2��������1�еõ�����Һ���ˣ���������ˮϴ����ϴ��Һ��ɫ | |
| ����3��ȥ����2�õ������������Թ��У��μ� | |
��ѡ�Լ���ϡ���ᡢ���Ƶ���ˮ��0.1mol��L-1CuSO4��Һ��20% KSCN��Һ������ˮ��
����ȤС���������в��ĵ���FeC2O4��2H2O���ȷֽ�ʱ�������������¶ȱ仯����������ͼ��ʾ��д�����ȵ�400��ʱ��FeC2O4��2H2O�������ȷֽ�Ļ�ѧ����ʽΪ��_______________
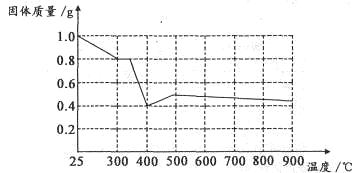
����ͼ������1.0g�������������������г��ڳ�ּ��ȣ����ղ�����ɫ�������������0.4g��ijͬѧ�ɴ˵ó����ۣ�����������������Ƿ�ͬ���ͬѧ�Ľ��ۣ����������ɣ�______________________��
ij̽��С�������ͼ��ʾװ�ý���Fe����ˮ�����ķ�Ӧ��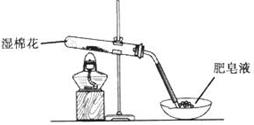
��1��ʵ��ǰ���װ�������Եķ���Ϊ________________________________________________________��
��2������ʵ�������������ʵ�������_____________________________________________��
��3����̽��С���Ϊ���飬����ͼװ�ý��жԱ�ʵ�飬�����þƾ���ơ������þƾ��Ƽ��ȣ���Ӧ�����Ϊ��ɫ��ĩ(������)������ֱ��ò����������ʵ�顣
| ���� | ���� | �������� | �������� |
| 1 | ȡ��ɫ��ĩ����ϡ���� | �ܽ⣬������ | �ܽ⣬������ |
| 2 | ȡ����1����Һ���μ�����KMnO4��Һ | ��ɫ��ȥ | ��ɫ��ȥ |
| 3 | ȡ����1����Һ���μ�KSCN��Һ | ��� | ������ |
| 4 | ����3��Һ�еμ�������ˮ | ��ɫ��ȥ | �ȱ�죬����ɫ |
������õ��ĺ�ɫ��ĩ�� ��
�ڼ��鲽��1�з�Ӧ�����ӷ���ʽΪ ��
�����鲽��4�У���Һ����ԭ��Ϊ ����Һ��ɫ���ܵ�ԭ���� ����֤����Ϊ ��
��������������Ԫ�أ�����ȱ����ƶѪ�ij��������Ƿ��ò���ҩ��������ơ�(��Ҫ�ɷ֣��������������ʰ���ɫ)���г���һ�ֳ����IJ���ҩ���ҩƷ������ˮ�������������е�θ�ᡣ
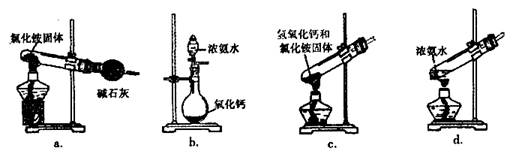
ijͬѧΪ�˼�⡰�����ơ�ҩƬ��Fe2���Ĵ��ڣ���Ʋ�����������ʵ�飺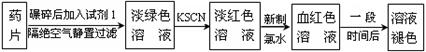
��1���Լ�1�� ������������ˮ����Һ�з��������ӷ�Ӧ����ʽ�ǣ� �� ��
��2������KSCN��Һ����δ��������ˮ������£���Һ��Ҳ�����˺�ɫ������ܵ�ԭ���� ��
��3����ʵ���з��ַ���һ��ʱ�䣬��Һ����ɫ������ȥ��Ϊ�˽�һ��̽����Һ��ɫ��ԭ�ס��ҡ�����λͬѧ���Ƚ����˲��룺
| ��� | �� �� |
| �� | ��Һ�еģ�3��Fe�ֱ���ԭΪ��2��Fe |
| �� | ��Һ�е�SCN������������ˮ���� |
| �� | ���Ƶ���ˮ����Ư���ԣ�������ҺƯ�� |
| ��� | ʵ����� | Ԥ������ͽ��� |
| �� | | |
| | | |
| | | |
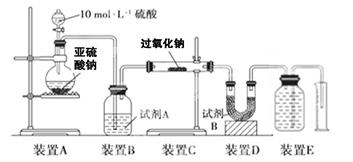
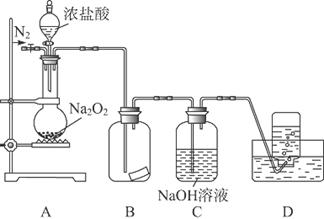
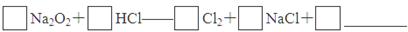


 �ķ����� ��
�ķ����� ��