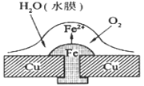
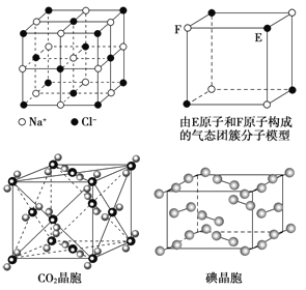
��Ŀ����
����Ŀ�����ڱ�ǰ�����ڵ�Ԫ��a��b��c��d��e��ԭ��������������a�����������������Ԫ�أ�c�ǵؿ��к�������Ԫ�أ�d��aͬ�壬e2+����3d�������9�����ӡ��ش��������⣺
��1��dԭ�ӵĻ�̬ԭ�ӵ����Ų�ʽΪ____________
��2������d������da������ac2���۵��ɸߵ��͵�˳��Ϊ__________ (�û�ѧʽ��ʾ����ͬ)��a��b��c����Ԫ�صĵ�һ�������ɴ�С��˳��Ϊ________________________��
��3��Ԫ��b��c�γ����ֳ���������bc2����bc3��������bc2�����ӵ�����ԭ���ӻ��������Ϊ____________��bc3�����ӵĿռ乹��Ϊ____________��
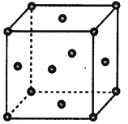
��4��e���ʵľ����ṹ��ͼ��ʾ��eԭ�Ӱ뾶Ϊrpm��e�����ܶȵļ���ʽΪ_________g��cm-3��(�ú�NA��r�ı���ʽ��ʾ)
���𰸡�[Ne]3s23p2 SiC>Si >CO2 N��O��C sp2 ƽ�������� 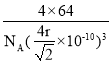 ����
����![]() ��
��
��������

��ϰ��ϵ�д�
�����Ŀ