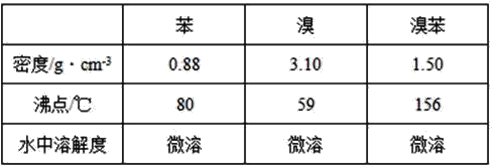
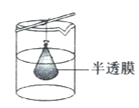
��Ŀ����
����Ŀ�������£����ȡ0.1mol/LHA��Һ��0.1mol/LNaOH��Һ��������(���Ի�Ϻ���Һ����ı仯)����û����Һ��pH��8���Իش��������⣺
��1�������Һ��pH��8��ԭ��(�����ӷ���ʽ��ʾ)��____��
��2�������Һ����ˮ�������c(H��)____0.1mol/LNaOH��Һ����ˮ�������c(H��)��(������������������������)
��3��������Һ��������ʽ�ľ�ȷ������(���������)��c(Na��)��c(A��)��____mol/L��c(OH��)��c(HA)��____mol/L��
��4����֪NH4A��ҺΪ���ԣ���֪HA��Һ�ӵ�Na2CO3��Һ��������ų������ƶ�(NH4)2CO3��Һ��pH_____7(��������������С��������������)����ͬ�¶��µ�Ũ�ȵ���������Һ��
A��NH4HCO3 B��NH4A C��(NH4)2SO4 D��NH4Cl
��pH�ɴ�С��˳�������ǣ�_____(�����)��
���𰸡�)A����H2O![]() HA��OH�� �� 9.9��10��7 10��8 ���� ABDC
HA��OH�� �� 9.9��10��7 10��8 ���� ABDC
��������
�����£������ʵ�����HA��NaOHǡ�÷�Ӧ����NaA�������Һ�ʼ��ԣ�˵��NaAΪǿ�������Σ���HAΪ���ᡣ
��1��A-ˮ���NaA��Һ�ʼ��ԣ�ˮ�ⷽ��ʽΪ��A-+H2OHA+OH-��
�ʴ�Ϊ��A-+H2OHA+OH-��
��2�����κ������������ܴٽ�ˮ���룬����������ǿ��������ˮ���룬���Ի����Һ����ˮ�������c��H+����0��1molL-1NaOH��Һ����ˮ�������c��H+����
�ʴ�Ϊ ����
��3���ɵ���غ��֪��c��Na+��-c��A-��=c��OH-��-c��H+��=10-6-10-8=9��9��10-7mol/L�� 0��1mol/L HA��Һ��0��1mol/L NaOH��Һ������������NaA��Һ����û����Һ��pH=8��c��H+��=10-8mol/L����Һ�Լ��ԣ�˵��A-����ˮ�⣬��Һ�д��������غ㣺c��OH-��=c��H+��+c��HA������c��OH-��-c��HA��=c��H+��=10-8mol/L��
�ʴ�Ϊ 9.9��10��7 ��10��8��
��4����HA��Һ�ӵ�Na2CO3��Һ��������ų���˵��HA�����Ա�̼���ǿ��NH4A��ҺΪ���ԣ� ˵����ͬ�����£���ˮ��HA�ĵ���̶���ͬ�����ԣ�NH4��2CO3��笠����ӵ�ˮ��̶�С��̼������ӵ�ˮ��̶ȣ�������Һ��pH��7����NH4��2SO4 ��NH4Cl��ǿ�������Σ�笠�����ˮ�����Һ�����ԣ���Һ��笠�����Ũ��Խ��ˮ��̶�ԽС����ˮ��ĸ����࣬�����Ȼ����Һ��pHֵ��������泥�NH4A��Һ���������ӵ�ˮ��̶���ȣ�������Һ�����ԣ���Һ��pHֵ�����Ȼ�泥�NH4HCO3��Һ��笠����ӵ�ˮ��̶�С��̼��������ӵ�ˮ��̶ȣ���Һ�ʼ��ԣ�������Һ��pH ֵ�����pH��С˳��ΪA��B��D��C��
�ʴ�Ϊ�����ڣ�ABDC��

����Ŀ��ú�����г����о���ͬ�¶���ƽ�ⳣ����Ͷ�ϱȼ���ֵ�����⡣��֪��CO(g)+H2O(g)H2(g)+CO2(g)ƽ�ⳣ�����¶ȵı仯���±���
�¶�/�� | 400 | 500 | 800 |
ƽ�ⳣ��K | 9.94 | 9 | 1 |
�Իش��������⣺
��1����������Ӧ��________��Ӧ (��������������������)��
��2�����������ĸı��ܼӿ��䷴Ӧ���ʵ���_______(ѡ�����)��
�������¶ȣ��ڱ���������䣬ֻ����CO���������۱���������䣬����Neʹ��ϵѹǿ���ܱ���ѹǿ���䣬����Neʹ�������������
��3����һ�ݻ�Ϊ2L���ܱ������ڣ�����0.2molCO��0.4molH2��������ӦCO(g)+2H2(g) CH3OH(g)��CO��ƽ��ת�������¶ȣ�ѹǿ�Ĺ�ϵ����ͼ��ʾ��
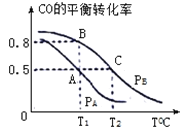
��A��B�����Ӧ��ѹǿ��С��ϵ��PA________PB��(����>������<������=��)
��A��B��C�����ƽ�ⳣ��KA��KB��KC�Ĵ�С��ϵ�� ____________��
����P1ѹǿ��T1��ʱ���÷�Ӧ��ƽ�ⳣ��K=___________��
��4��T1�桢1L���ܱ������ڷ���������Ӧ�����ijʱ�̸����ʵ����ʵ������£�CO��0.1mol H2��0.2mol CH3OH��0.2mol����ʱv�� ____ v��(�� >��< �� =)��