��Ŀ����
5������Ч�ļ״�ȼ�ϵ�ز��ò�Ϊ�缫���ϣ����缫�Ϸֱ�ͨ��CH3OH��O2��ij�о�С�齫�����״�ȼ�ϵ�ش�������Ϊ��Դ�����б����Ȼ�����Һ���ʵ�飬��ͼ��ʾ���ش��������⣺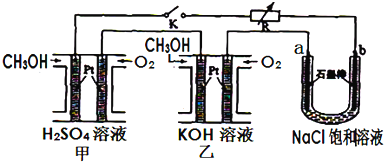
��1����ȼ�ϵ�صĸ�����ӦʽΪ2CH3OH-12e-+16OH-=2CO32-+12H2O��
��2����ԭNaCl��ҺΪ300ml�������������������䣬��ÿ��ȼ�ϵ����ͨ��0.16g�״���ȫ��Ӧʱ������Һ��PHΪ13��
���� ��1��CH3OH����ȼ�ϵ���У�����CH3OHʧ���ӱ�������
��2�����ݹ�ϵʽ1molCH3OH��6mol e-��3mol Cl2��6molOH-���㣮
��� �⣺��1���ڼ�����Һ�У�CH3OH����ȼ�ϵ���У�����CH3OHʧ���ӱ���������ӦʽΪ��2CH3OH-12e-+16OH-=2CO32-+12H2O��
�ʴ�Ϊ��2CH3OH-12e-+16OH-=2CO32-+12H2O��
��2������2CH3OH-12e-+16OH-=2CO32-+12H2O�ã���ϵʽ1molCH3OH��6mol e-��3mol Cl2��6molOH-
1 mol 6mol
$\frac{0.16}{32}$=0.005mol n��OH-��
����n��OH-��=0.03mol
����c��OH-��=$\frac{0.03}{0.3}$=0.1mol/L������c��H+��=$\frac{1��10{\;}^{-14}}{0.1}$=10-13��
���ԣ�pH=13���ʴ�Ϊ��13��
���� ���⺭�ǵ��غ�ԭ��ص��������ݣ��漰�缫�ж���缫��Ӧʽ��д�����⣬����ʱע���������ԭ�ĽǶ��ж�ԭ��ص��������Լ��缫����ʽ����д���������ѵ���״���Ϊ�缫����ʽ����д��

 ����С����ͬ������ϵ�д�
����С����ͬ������ϵ�д�| A�� | c ��Cl-����c ��NH4+����c ��OH-����c ��H+�� | B�� | c ��NH4+����c ��Cl-����c ��OH-����c ��H+�� | ||
| C�� | c ��NH4+����c ��Cl-����c ��H+����c ��OH-�� | D�� | c ��Cl-����c ��NH4+����c ��H+����c ��OH-�� |
| A�� | �ڹ���������ˮ�ķ�Ӧ�У�ÿ����1mol������ת�Ƶ��ӵ���ĿΪ1NA | |
| B�� | ��0.1mol NaClȫ�������Ҵ����Ƴɽ��壬���к��еĽ���������ĿΪ0.1NA | |
| C�� | ��״���£�22.4LCCl4����NA��CCl4���� | |
| D�� | 1 molOD- ���е����ӡ���������Ϊ9NA |
| A�� | 17.4g | B�� | 46.8g | C�� | 40.8g | D�� | 23.4g |
| A�� | ���ߵĵ����ڳ����¶�������Ũ�����Ũ���� | |
| B�� | ���ߵĵ��ʷ����ڿ����о�ֻ���������� | |
| C�� | ��������Ӧ������������ˮ�ж������ܽ�ƽ�� | |
| D�� | ��ҵ���Ʊ������ֽ����ķ�������Ϊ��ⷨ���ȷֽⷨ���Ȼ�ԭ�� |
 ��֪��
��֪��| ҩƷ���� | �۵�/�� | �е㣨�棩 | �ܶ�g/cm3 | �ܽ��� |
| ���� | -89.5 | 117.7 | 0.8098 | ����ˮ������Ũ���� |
| 1-�嶡�� | -112.4 | 101.6 | 1.2760 | ������ˮ��Ũ���� |
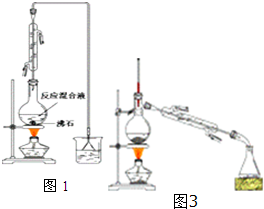
��һ���Ʊ�1-�嶡��ֲ�Ʒ����ͼ1װ�õ�Բ����ƿ�����μ���NaBr��10mL��������2����ʯ����������1��1��������Һ��ҡ�ȣ�����30min����ѧ����ʽ��NaBr+H2SO4+CH3CH2CH2CH2OH��CH3CH2CH2CH2Br+NaHSO4+H2O
��1����Ӧװ���м����ʯ��Ŀ���Ƿ�ֹҺ�屩�У����������Ϊ1��1���������õĶ�������Ϊb��ѡ���ţ�
a����ƽ b����Ͳ c������ƿ d���ζ���
��2�������ܵĽ�ˮ����Ϊ�½��ϳ�������ѡ���ԭ�����ܸ����������
��3��ͼ2װ���У��ܴ�����ͼ������ռ�װ�õ���ABD������ţ���

��4������Ũ�������ʵ�飬�л����л�����ػ�ɫ����ȥ�������ʵ���ȷ������d��ѡ���ţ���
a������ b������������Һϴ�� c�������Ȼ�̼��ȡ d��������������Һϴ��
�������Ʊ���Ʒ�����õ��Ĵ�1-�嶡��������Ũ���ᡢˮ��10% ̼���ơ�ˮϴ�Ӻ������ˮ�Ȼ��ƽ��и��Ȼ���ٽ�1-�嶡�鰴ͼ3װ������5���ռ���Ʒʱ�����Ƶ��¶�Ӧ��101.6�����ң�
��6��ʵ���Ƶõ�1-�嶡�������Ϊ10.895g�����������IJ���Ϊ72.7%��
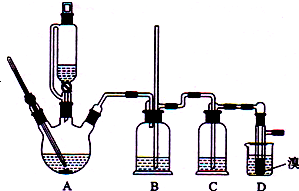 ʵ�����Ʊ�1��2-�������飨��ɫҺ�壬�۵�9�棬�ܶ�2.2g•cm-3���ķ�Ӧԭ�����£�
ʵ�����Ʊ�1��2-�������飨��ɫҺ�壬�۵�9�棬�ܶ�2.2g•cm-3���ķ�Ӧԭ�����£�