题目内容
已知:

(1)Zn(s)+1/2O2(g) = ZnO(s) △H =-348.3 kJ/mol
 (2) 2Ag(s)+1/2O2(g) = Ag2O(s) △H =-31.0 kJ/mol
(2) 2Ag(s)+1/2O2(g) = Ag2O(s) △H =-31.0 kJ/mol
 则Zn(s)+Ag2O(s) = ZnO(s)+2Ag(s)的△H等于( )
则Zn(s)+Ag2O(s) = ZnO(s)+2Ag(s)的△H等于( )


(1)Zn(s)+1/2O2(g) = ZnO(s) △H =-348.3 kJ/mol
 (2) 2Ag(s)+1/2O2(g) = Ag2O(s) △H =-31.0 kJ/mol
(2) 2Ag(s)+1/2O2(g) = Ag2O(s) △H =-31.0 kJ/mol 则Zn(s)+Ag2O(s) = ZnO(s)+2Ag(s)的△H等于( )
则Zn(s)+Ag2O(s) = ZnO(s)+2Ag(s)的△H等于( )
| A.-317.3 kJ/mol | B.-379.3 kJ/mol |
| C.-332.8 kJ/mol | D.317.3 kJ/mol |
A
考查反应热的计算,根据盖斯定律求解即可
①-②得:Zn(s)+Ag2O(s) = ZnO(s)+2Ag(s) △H =-317.3kJ/mol,故答案为A
①-②得:Zn(s)+Ag2O(s) = ZnO(s)+2Ag(s) △H =-317.3kJ/mol,故答案为A

练习册系列答案
 学期复习一本通学习总动员期末加暑假延边人民出版社系列答案
学期复习一本通学习总动员期末加暑假延边人民出版社系列答案 芒果教辅暑假天地重庆出版社系列答案
芒果教辅暑假天地重庆出版社系列答案
相关题目
 。若加入少量醋酸钠固体,则CH3COOH
。若加入少量醋酸钠固体,则CH3COOH CH3COO-+H+向左移动,α减小,Ka变小
CH3COO-+H+向左移动,α减小,Ka变小 2Fe(s)+3CO(g),△H=+489.0 kJ/mol。
2Fe(s)+3CO(g),△H=+489.0 kJ/mol。 Si(s)+4HCl(g),计算该反应的反应热△H为 。
Si(s)+4HCl(g),计算该反应的反应热△H为 。 全部由碳燃烧提供,问理论上要消耗多少克碳?
全部由碳燃烧提供,问理论上要消耗多少克碳?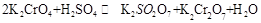
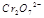 在水溶液中为红色,
在水溶液中为红色,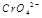 在水溶液中为黄色。某条件下该反应建立平衡后,体系为两种离子的混合液,颜色为橙色。
在水溶液中为黄色。某条件下该反应建立平衡后,体系为两种离子的混合液,颜色为橙色。 ;
;
 ,能有效地利用资源,并减少空气中的温室气体。工业上正在研究利用
,能有效地利用资源,并减少空气中的温室气体。工业上正在研究利用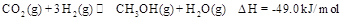
 充入一容积为2L的密闭容器中,测得H
充入一容积为2L的密闭容器中,测得H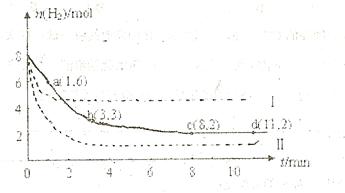
 2SO3(g) △H。测得SO2的转化率为90%,则在此条件下,反应放出的热量为 ( )
2SO3(g) △H。测得SO2的转化率为90%,则在此条件下,反应放出的热量为 ( )