��Ŀ����
����ʯ����Ҫ�ɷ�ΪK2SO4��Al2(SO4)3��2Al2O3��6H2O�������������Fe2O3���ʡ�ijУ�о�С����������ʯ�Ʊ������������������£�
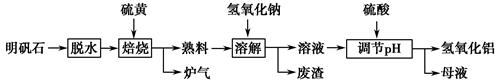
(1)�����ա������з�Ӧ�Ļ�ѧ����ʽΪ Al2(SO4)3��
Al2(SO4)3�� S
S
 Al2O3��
Al2O3�� ________����
________����
(2)������Һ�ͷ����IJ�����________�����ܽ⡱ʱ��Ӧ�����ӷ���ʽΪ_________________________________��
(3)������pH������ˡ�ϴ��Al(OH)3������֤��������ϴ�Ӹɾ���ʵ�������������________��
(4)��ĸҺ���пɻ��յ�������________��
(5)�������ա�������ǡ������48 g��ƣ������������տɵõ�________ g����������

(1)�����ա������з�Ӧ�Ļ�ѧ����ʽΪ
 Al2(SO4)3��
Al2(SO4)3�� S
S
 Al2O3��
Al2O3�� ________����
________����(2)������Һ�ͷ����IJ�����________�����ܽ⡱ʱ��Ӧ�����ӷ���ʽΪ_________________________________��
(3)������pH������ˡ�ϴ��Al(OH)3������֤��������ϴ�Ӹɾ���ʵ�������������________��
(4)��ĸҺ���пɻ��յ�������________��
(5)�������ա�������ǡ������48 g��ƣ������������տɵõ�________ g����������
��(1)2��3��2��9��SO2��(2)���ˡ�Al2O3��2OH��===2AlO2����H2O��(3)ȡ���һ��ϴ�ӵ���Һ���Թ��У��μ�BaCl2��Һ�����ް�ɫ�������ɣ���֤����ϴ�Ӹɾ���(4)K2SO4��Na2SO4��(5)468
��(1)������֪�ķ�Ӧ����������֪��ֻ����Ԫ�ػ��ϼ۷����仯��������ֻ��������SO2��(2)������̿�֪��������Һ�ͷ������ù��˲������ܽ�ʱ��Al2O3��Ӧ��(3)Al(OH)3��������������SO42�����ʿ�ȡ���һ��ϴ�ӵ���Һ���Թ��У��μ�BaCl2��Һ�����ް�ɫ����������֤����ϴ�Ӹɾ���(4)��ĸҺ���пɻ��յ����������ܵ�K2SO4��Na2SO4��(5)���ݷ�Ӧ�Ļ�ѧ����ʽ��֪��2Al2(SO4)3��3S��n(S)��1.5 mol��n[Al2(SO4)3]��1 mol��n(Al2O3)��1 mol������ʯ��n(Al2O3)��2 mol��������Ԫ���غ��֪��n(Al)��n[Al(OH)3]��6 mol����������������Ϊ468 g��

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ

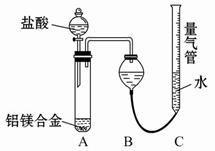
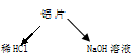
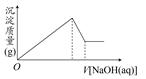
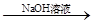 �ⶨʣ�����������ʵ���з�����Ӧ�Ļ�ѧ����ʽ�ǣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߡ�
�ⶨʣ�����������ʵ���з�����Ӧ�Ļ�ѧ����ʽ�ǣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߡ�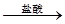 �ⶨ������������ʵ��װ�ã�
�ⶨ������������ʵ��װ�ã�