��Ŀ����
����Ŀ��ijѧ����һ֧�Թ��ﰴһ��˳��ֱ�������м������ʣ�A��KI��Һ��B��������Һ��C��NaOH��Һ��D����ˮ��������Һ��ɫ������˳��仯������ɫ�����ػ�ɫ������ɫ������ɫ��������Һ��ɫ�ı仯�ش��������⣺
��1����������ҩƷ��˳���� ��
��2��д���١��ڵ����ӷ���ʽ����Ϊ������ԭ��Ӧ�����������ת�Ƶķ������Ŀ�� ��
��3��д���ۡ��ܵĻ�ѧ����ʽ�� ��
���𰸡�
��1��A��D��B��C
��2��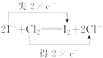
��3��I2+2NaOH�TNaI+NaIO+H2O
���������⣺����Һ��ɫ������˳��仯������ɫ�����ػ�ɫ������ɫ������ɫ��֪����Һ���ػ�ɫ��˵����Һ����I2���ɣ���ˮ��KI��Һ��Ӧ����I2 �� ����ˮ��dz��ɫ���ʢ�Ϊ����KI��Һ����Ϊ������ˮ����Һ����ɫ��I2�������γɵģ��ʢ�Ϊ���������Һ��������I2+2NaOH�TNaI+NaIO+H2O������NaOH��Һ��I2����ʧ����ɫ����Ϊ��ɫ���ʢ�Ϊ����NaOH��Һ����1��������������֪����������ҩƷ��˳����A��D��B��C�����Դ��ǣ�A��D��B��C����2����ˮ��KI��Һ��Ӧ����I2 �� Ϊ������ԭ��Ӧ�������ӷ�Ӧ������ת�Ƶķ������ĿΪ  �����Դ��ǣ�
�����Դ��ǣ�  ����3���ۡ��ܵĻ�ѧ����ʽΪI2+2NaOH�TNaI+NaIO+H2O�����Դ��ǣ�I2+2NaOH�TNaI+NaIO+H2O��
����3���ۡ��ܵĻ�ѧ����ʽΪI2+2NaOH�TNaI+NaIO+H2O�����Դ��ǣ�I2+2NaOH�TNaI+NaIO+H2O��

����Ŀ���������ʷ��������ȷ���ǣ� ��
A | B | C | D | |
ǿ����� | HBr | FeCl3 | H3PO4 | Ca(OH)2 |
������� | HF | CH3COOH | BaSO4 | HCl |
�ǵ���� | CCl4 | Cu | H2O | C2H5OH |
A. A B. B C. C D. D
����Ŀ����ˮ��Һ�д�������ƽ�⣺I2(aq)��I��(aq)=I3-(aq)����ò�ͬ�¶��¸÷�Ӧ��ƽ�ⳣ��K�����ʾ������˵����ȷ����
t/�� | 5 | 15 | 25 | 35 | 50 |
K | 1100 | 841 | 689 | 533 | 409 |
A. ��ӦI2(aq)��I��(aq)![]() I3-(aq)����H>0
I3-(aq)����H>0
B. 25��ʱ������Һ�м�������KI���壬ƽ�ⳣ��KС��689
C. �����������䣬�����¶ȣ���Һ��c( I3-)��С
D. �÷�Ӧ��ƽ�ⳣ������ʽΪK��c��I-��/c��I3-��
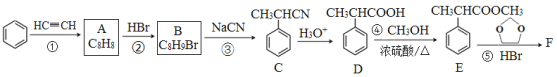
����Ŀ�����ڹ�ũҵ������Ӧ�ù㷺����ѹǿΪ30MPaʱ���ϳɰ�ƽ����������NH3������������±���
�¶�/�� | 200 | 300 | 400 | 500 | 600 |
������/% | 89.9 | 71.0 | 47.0 | 26.4 | 13.8 |
��ش�
(1)���ݱ������ݣ���ϻ�ѧƽ���ƶ�ԭ����˵���ϳɰ���Ӧ�Ƿ��ȷ�Ӧ��ԭ����_______��
(2)��һ���¶��£���2molN2��6molH2ͨ�뵽���Ϊ2L���ܱ������У�������ӦN2��3H2![]() 2NH3��2min�ﵽƽ��״̬ʱ��H2ת������50%�����¶��µ�ƽ�ⳣ��K��_______________(�������������ʾ)����ʹK�����Բ�ȡ�Ĵ�ʩ��______��
2NH3��2min�ﵽƽ��״̬ʱ��H2ת������50%�����¶��µ�ƽ�ⳣ��K��_______________(�������������ʾ)����ʹK�����Բ�ȡ�Ĵ�ʩ��______��
(3)�ӻ�ѧƽ���ƶ��ĽǶȷ��������H2ת���ʿ��Բ�ȡ�Ĵ�ʩ��______(ѡ�������ĸ)
a����ʱ�����NH3 b�������¶� c������ѹǿ d��ʹ�ô���
(4)NH3�ֽܷ�ΪN2��H2������ͬ�����£���÷ֽ���������ܶ�Ϊ�ֽ�ǰ��2/3���ķֽ���Ϊ_____________��