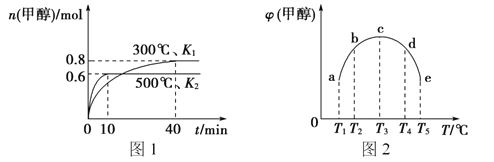
��Ŀ����
����Ŀ�������£���0.01mol/LNaOH��Һ�ζ�20.00mL0.01mol/LCH 3COOH��Һ�����õζ���������ͼ������˵����ȷ����
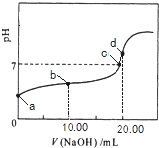
A. b���Ӧ����Һ��: c(OH��)+c(CH3COO��) = c(Na+)+c(H+)
B. a���Ӧ��Һ��pH= 2
C. C���ʾNaOH��Һ��CH3COOH��Һǡ����ȫ��Ӧ
D. d���Ӧ����Һ�У�ˮ�ĵ���̶�С��ͬ���´�ˮ�ĵ���̶�
���𰸡�A
��������A���ɵ���غ��֪��c(OH-)+c(CH3COO-)=c(Na+)+c(H+)��ѡ��A��ȷ��B��CH3COOH�����ᣬ����ȫ���룬��0.01mol/LCH3COOH��ҺpH>2��a���Ӧ��Һ��pH>2��ѡ��B����C��c���ʾNaOH��Һ��CH3COOH��Һ��Ӧ����Һ�����ԣ����ỹû��ȫ��Ӧ��ѡ��C����D��d���Ӧ����ҺΪ��ȫ��Ӧ��Ĵ�������Һ�������Ƶ�ˮ��ٽ�ˮ�ĵ��룬�ʸõ���ˮ�ĵ���̶ȴ���ͬ���´�ˮ�ĵ���̶ȣ�ѡ��D����ѡA��

��ϰ��ϵ�д�
�����Ŀ