��Ŀ����
15��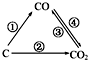 �������繤ҵ���õķ�չ���˿ڵľ�����ȫ����Դ���ż�������������Խ��Խ���ص����⣬��ν��ʹ�����CO2�ĺ�������Ч�ؿ�������CO2������ȫ������ձ����ӣ�
�������繤ҵ���õķ�չ���˿ڵľ�����ȫ����Դ���ż�������������Խ��Խ���ص����⣬��ν��ʹ�����CO2�ĺ�������Ч�ؿ�������CO2������ȫ������ձ����ӣ���1����ͼΪC����������ı仯��ϵͼ�����ٱ仯���û���Ӧ�����仯ѧ����ʽ��ΪC+H2O$\frac{\underline{\;����\;}}{\;}$CO+H2��ͼ�б仯������Щ�����ȷ�Ӧ�٢ۣ�����ţ���
��2����ú��Ϊȼ�Ͽ�ͨ����������;����
;����C��s��+O2��g���TCO2��g����H1��0����
;�������Ƴ�ˮú����C��s��+H2O��g���TCO��g��+H2��g����H2��0����
��ȼ��ˮú����2CO��g��+O2��g���T2CO2��g����H3��0����
2H2��g��+O2��g���T2H2O��g����H4��0����
��;����ų����������ڣ�����ڡ��������ڡ���С�ڡ���;����ų�����������H1����H2����H3����H4����ѧ��ϵʽ�ǡ�H1=��H2+$\frac{1}{2}$����H3+��H4����
��3���״���һ�ֿ�������Դ�����п�����Ӧ�õĹ���ǰ������ҵ�Ͽ������·����ϳɼ״���
����һ��CO��g��+2H2��g��?CH3OH��g�� ��������CO2��g��+3H2��g��?CH3OH��g��+H2O��g��
��25�桢101kPa�£�1�˼״���ȫȼ�շ���22.68kJ��д���״�ȼ�յ��Ȼ�ѧ����ʽ��CH3OH��l��+$\frac{3}{2}$O2��g��=CO2��g��+2H2O��l����H=-32akJ•mol-1��
��4��������ұ������������һ����Ӧ�ǽ�ԭ�Ͻ��ʯת����
TiO2�����ʯ��+2C+2Cl2$\frac{\underline{\;����\;}}{\;}$TiCl4+2CO
��֪��C��s��+O2��g���TCO2��g����H=-393.5kJ•mol-1
2CO��g��+O2��g���T2CO2��g����H=-566kJ•mol-1
TiO2��s��+2Cl2��g���T��s��+O2��g����H=+141kJ•mol-1
��TiO2��s��+2Cl2��g��+��s���T��s��+��g���ġ�H=-80kJ/mol��
��5�����������ھ�������������ˮ������������ҵ�������ΪƯ�����������������������ҿ������������е��ʷ�Ӧ����
��s��+O3��g���T3Ag2O��s����H=-235.8kJ•mol-1��
��֪��2Ag2O��s���T4Ag��s��+O2��g����H=+62.2kJ•mol-1��
��O3ת��ΪO2���Ȼ�ѧ����ʽΪ2O3��g���T3O2��g����H=-285kJ/mol��
���� ��1������̼���仯�������ʷ���ת����ϵ�������û���Ӧ������̼��ˮ������Ӧ����һ����̼��������������������ﷴӦ������̼��ˮ������Ӧ��̼�Ͷ�����̼��ӦΪ���ȷ�Ӧ��
��2�����ݸ�˹���ɵ�ԭ�����жϣ����ݸ�˹���ɢ�=��+�ۡ�$\frac{1}{2}$+�ܡ�$\frac{1}{2}$�жϣ�
��3������ȼ���ȸ�����1mol��ȼ����ȫȼ�������ȶ�������ų����������������������32g�״�ȼ�����ɶ�����̼��Һ̬ˮ���ȣ�����Ȼ�ѧ����ʽ��д��������ע���ʾۼ�״̬�Ͷ�Ӧ�ʱ䣻
��4�����ݸ�˹���ɢ��2-��+����㣻
��5����6Ag��s��+O3��g���T3Ag2O��s������H=-235.8kJ•mol-1��
��2Ag2O��s���T4Ag��s��+O2��g������H=+62.2kJ•mol-1��
���ݸ�˹���ɿ�֪���2+���3�ɵõ���2O3��g���T3O2��g�����Դ˼��㷴Ӧ�ȣ�
��� �⣺��1�������û���Ӧ������̼��ˮ������Ӧ����һ����̼��������������������ﷴӦ����Ӧ�Ļ�ѧ����ʽΪ��C+H2O$\frac{\underline{\;����\;}}{\;}$CO+H2������̼��ˮ������Ӧ��̼�Ͷ�����̼��ӦΪ���ȷ�Ӧ���٢�Ϊ���ȷ�Ӧ��
�ʴ�Ϊ��C+H2O$\frac{\underline{\;����\;}}{\;}$CO+H2���٢ۣ�
��2���ɸ�˹���ɿ�֪������һ����Ӧ���Էֲ����У��������Ӧ�����ջ�ų��������ܺ��������Ӧһ�η���ʱ���ջ�ų���������ͬ��
���ݸ�˹���ɣ���=��+�ۡ�$\frac{1}{2}$+�ܡ�$\frac{1}{2}$ ���ԡ�H1=��H2+$\frac{1}{2}$����H3+��H4�����ʴ�Ϊ�����ڣ���H1=��H2+$\frac{1}{2}$����H3+��H4����
��3����25�桢101kPa�£�1g�״���CH3OH��ȼ������CO2��Һ̬ˮʱ����akJ��32g�״�ȼ�����ɶ�����̼��Һ̬ˮ�ų�����Ϊ32QKJ�����ʾ�״�ȼ���ȵ��Ȼ�ѧ����ʽΪ��CH3OH��l��+$\frac{3}{2}$O2��g��=CO2��g��+2H2O��l����H=-32akJ•mol-1��
�ʴ�Ϊ��CH3OH��l��+$\frac{3}{2}$O2��g��=CO2��g��+2H2O��l����H=-32akJ•mol-1��
��4����C��s��+O2��g���TCO2��g����H=-393.5kJ•mol-1��
��2CO��g��+O2��g���T2CO2��g����H=-566kJ•mol-1
��TiO2��s��+2Cl2��g���TTiCl4��s��+O2��g����H=+141kJ•mol-1
��˹���ɼ�����2-��+��õ���TiO2��s�����ʯ��+2C��s��+2Cl2��g��=TiCl4��s��+2CO��g����H=-80 kJ/mol��
�ʴ�Ϊ��-80 kJ/mol��
��5����6Ag��s��+O3��g���T3Ag2O��s������H=-235.8kJ•mol-1��
��2Ag2O��s���T4Ag��s��+O2��g������H=+62.2kJ•mol-1��
���ݸ�˹���ɿ�֪���2+���3�ɵõ���2O3��g���T3O2��g������Ӧ�ȡ�H=��-235.8kJ•mol-1����2+��+62.2kJ•mol-1����3=-285kJ/mol��
�ʴ�Ϊ��2O3��g���T3O2��g����H=-285kJ/mol��
���� ���⿼����ۺϣ��漰�Ȼ�ѧ��Ӧ����ʽ����˹���ɵ�Ӧ�ã����ط�Ӧԭ���Ŀ��飬ע��֪ʶ��Ǩ��Ӧ�ã���Ŀ�Ѷ��еȣ�

| A�� | �������ӣ�����������Ų���ȫ��ͬ�����仯ѧ����һ����ͬ | |
| B�� | ����ԭ���γɵ����ӣ�һ������ϡ������Ԫ��ԭ�ӵĺ�������Ų� | |
| C�� | ��ԭ�ӣ������������Ų���ͬ����һ������ͬ��Ԫ�� | |
| D�� | �����ӵĺ�������Ų�һ�������ԭ������С��ϡ������Ԫ��ԭ�ӵĺ�������Ų���ͬ |
| A�� | �ö��Ե缫��������Ȼ��ƣ�2Cl-+2H2O$\frac{\underline{\;���\;}}{\;}$Cl2��+H2��+2OH- | |
| B�� | ������������Һ��ȥ�����������Ĥ��Al2O3+2OH-+3H2O�T2[Al��OH��4]- | |
| C�� | ��NaHCO3��Һ�м�������ij���ʯ��ˮ�����ְ�ɫ����2HCO${\;}_{3}^{-}$+Ca2++2OH-�TCaCO3��+CO${\;}_{3}^{2-}$+2H2O | |
| D�� | ��ʳ�׳�ȥˮƿ�е�ˮ����CO${\;}_{3}^{2-}$+2CH3COOH�T2CH3COO-+CO2��+H2O |
| A�� | ��Һ�ȿ��ø�ƿװ������Ӧ�� ��־��װ��Ũ����������۹���Ӧ�� ��־��װ��Ũ����������۹���Ӧ�� ��־ ��־ | |
| B�� | �����Ͻ��ڴ��Ž��յ�������ú��¯ȡů����Ϊ���ɵ�CO��CO2�����������ж� | |
| C�� | �����ͳ��������� �ܻ��ʹ�ã���Ϊ�ᷴӦ����Cl2�����ж� | |
| D�� | ����θ����Ļ�������θ����࣬���ó��ڴ������ú�Al��OH��3��θҩ |
| A�� | AgNO3 ��Һ�м���Cu��Cu+Ag+=Cu2++Ag | |
| B�� | NaHCO3��Һ�м���CH3COOH��CO32-+2CH3COOH=CO2��+2CH3COO-+H2O | |
| C�� | ��0.2mol FeBr2 ����Һ��ͨ��0.2mol Cl2��4Fe2++6Br-+5Cl2=4Fe3++3Br2+10Cl- | |
| D�� | ����������ʵ���Ũ�ȵ�NaHCO3��Ba��OH��2��Һ��ϣ�HCO3-+Ba2++OH-=BaCO3��+H2O |
| A�� | �϶������ڵ�������Fe3+��Cu2+��CO32-��Br- | |
| B�� | �϶����ڵ�������K+��SO42-��SO32- | |
| C�� | ��ȷ��ԭ��Һ���Ƿ����Cl-��CO32- | |
| D�� | ������ܸ���BaCl2������Ļ����Һ�������Һ�����ӵ��ж�Ҳ��Ӱ�� |