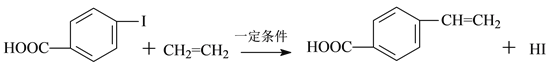��Ŀ����
����Ŀ��ij��ѧ��ȤС��Ϊ̽��SO2�Ļ�ѧ���ʣ����������ʵ��װ�á�
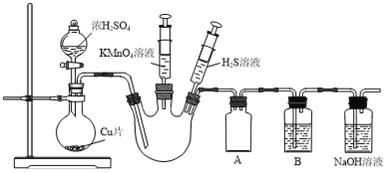
��1����װ������д��ڵ�������___________________��
��2��װ��A��������___________________��
��3���ٵ�������ƿ�г���SO2���壬֤��SO2���������Ե�ʵ�������������___________________��
����Ҫ֤��SO2����Ư���ԣ�����ϴ��ƿB�м���___________________��Һ��
��4��NaOH��Һ��������β���е�SO2�����ڿ�����������Һ�п��ܻ����SO![]() ��������Һ���Ƿ����SO
��������Һ���Ƿ����SO![]() ��ʵ�鷽����___________________��
��ʵ�鷽����___________________��
���𰸡� Cu��ŨH2SO4�ķ�Ӧȱ�ټ���װ�� ��ֹ���� �����ƣ���װ��H2S��Һע�����Ļ�����ע������������ƿ���г��ֻ������� Ʒ����Һ ȡ��������β�������Һ���Թ��У���������ϡ���ᣬ�ټ���BaCl2��Һ�������ְ�ɫ��������˵������SO![]() ������������������SO
������������������SO![]()
����������1��Cu��ŨH2SO4�ķ�Ӧ��Ҫ���ȣ�����װ�������ȱ�ټ���װ�á�
��2�����巢��װ�����ɵ�SO2�������������ƿ�У���������ƿ�Ҳർ����ֱ����Bװ�������������ײ�����������Aװ�õ������Ƿ�ֹ������
��3������������ƿ���ӵ�����һ��ע������ʢ��H2S��Һ����������ƿ�г���SO2���壬������SO2��H2S�ķ�Ӧ֤������������ԣ�ʵ������������ǣ�����װ��H2S��Һע�����Ļ�����ע�����г��ֻ��������ƶ�װ��H2S��Һע�����Ļ�����������ƿ�г��ֻ�����������SO2����ʹƷ����Һ��ɫ����������Ư���ԣ�������Ҫ֤��SO2����Ư���ԣ�����ϴ��ƿB�м���Ʒ����Һ��
��4������֪����Һ�к���SO32-��Ҫ�����Ƿ���SO42-�����ų����ţ�����ʵ�鷽��Ϊ��ȡ��������β�������Һ���Թ��У���������ϡ���ᣬ�ټ���BaCl2��Һ�������ְ�ɫ��������˵������SO42-�����������ɣ�����SO42-��

����Ŀ�������£���ijһԪ��HA��NaOH��Һ�������ϣ�ʵ����Ϣ���£�
ʵ���� | c(HA)mol��L��1 | c(NaOH)/mol��L��1 | ��Ӧ����ҺpH |
�� | 0.1 | 0.1 | pH=9 |
�� |
| 0.2 | pH=7 |
�����жϲ���ȷ���ǣ� ��
A. 0.1![]() ��HA��Һ����ˮ�������
��HA��Һ����ˮ�������![]()
B. ![]() һ������0.2
һ������0.2![]()
C. ��Ӧ�����Һ�У�HAռ��A��������0.02��
D. �ҷ�Ӧ����Һ�У�c(Na��)��)