��Ŀ����
7�������״���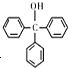 ����һ����Ҫ�Ļ���ԭ�Ϻ�ҽҩ�м��壬ʵ���Һϳ������״��ĺϳ�������ͼ1��ʾ��װ����ͼ2��ʾ
����һ����Ҫ�Ļ���ԭ�Ϻ�ҽҩ�м��壬ʵ���Һϳ������״��ĺϳ�������ͼ1��ʾ��װ����ͼ2��ʾ��֪����i�������Լ�����ˮ�⣬
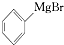 +H2O��
+H2O�� +M��OH��Br����ʽ�廯þ��
+M��OH��Br����ʽ�廯þ������������ʵ������������£�
| ���� | �۵� | �е� | �ܽ��� |
| �����״� | 164.2�� | 380�� | ������ˮ�������Ҵ������ѵ��л��ܼ� |
| ���� | -116.3�� | 34.6�� | ����ˮ�������Ҵ��������л��ܼ� |
| �屽 | -30.7�� | 156.2�� | ������ˮ�������Ҵ������ѵȶ����л��ܼ� |
| ���������� | -34.6�� | 212.6�� | ������ˮ |
| Mg��OH��Br | ������Ϊ���� | ������ˮ�������Ҵ����ѵȶ����л��ܼ� | |
�й̶��۵㣮
��ش��������⣺
��1��ͼ2�в�������B�����ƣ������ܣ�װ����ˮCaCl2������A�������Ƿ�ֹ�����е�ˮ��������װ�ã���������Լ�ˮ�⣮
��2��ͼ2�еμ�Һ��δ����ͨ��Һ©�����ú�ѹ��Һ©����������ƽ��ѹǿ��ʹ©����Һ��˳�����£���ȡ�����Լ�ʱҪ�����У����Բ���ˮԡ���ȷ�ʽ��
��3���Ƶõ������״��ֲ�Ʒ�У��������ѡ��屽���������������л���ͼ�ʽ�廯þ�����ʣ�������������ᴿ����������д���¿հף�
���У��ٲ���Ϊ����������ϴ��Һ���ѡ��a��������ѡ����ѡ��
A��ˮ B������ C���Ҵ� D����
�����Ʒ�Ѿ�ϴ�Ӹɾ��IJ���Ϊ��ȡ�������һ��ϴ��Һ���Թ��У��μ���������Һ�����������ɣ����Ѿ�ϴ�Ӹɾ�����֮��δϴ�Ӹɾ���
��4�����Ȳⶨ����ȡ2.60g��Ʒ�����������Һ���������������ƣ��������Ʋ���Ӧ������ַ�Ӧ������ɵ������ڱ�״���µ����Ϊ100�� 80mL�����Ʒ�������״�����������Ϊ90%��
���� ��1��ͼ�в�������B�������������ܣ����ڸ����Լ�����ˮ�⣬B�������Ƿ�ֹ�����е�ˮ��������װ�ã�
��2��װ���еμ�Һ��δ����ͨ��Һ©�����õ�Һ©����������ƽ��ѹǿ������ˮԡ���ȣ����Ⱦ��ȣ����ڿ����¶ȣ�
��3�������״��ֲ�Ʒ�к������ѡ��屽���������������л�������״��ķе���ߣ�������������ķ�����ȥ�л����ʣ���ʽ�廯þ����ˮ���������л��ܼ�������ϴ��Һѡ��ˮ�����ϴ���Ƿ�ɾ���ȡ�������һ��ϴ��Һ���Թ��У�����������Һ�����Ƿ��������ӣ�
��4����2-OH��H2�ɼ���������״������ʵ������ٸ���m=nM���������״������������������Ʒ�������״�������������
��� �⣺��1��ͼ�в�������B�������������ܣ����ڸ����Լ�����ˮ�⣬B�������Ƿ�ֹ�����е�ˮ��������װ�ã������ʽ�Լ�ˮ�⣬
�ʴ�Ϊ�������ܣ���ֹ�����е�ˮ��������װ�ã���������Լ�ˮ�⣻
��2��װ���еμ�Һ��δ����ͨ��Һ©�����õ�Һ©����������ƽ��ѹǿ��ʹ©����Һ��˳�����£�����ˮԡ���ȣ����Ⱦ��ȣ����ڿ����¶ȣ�����ˮԡ���ȣ����Ⱦ��ȣ����ڿ����¶ȣ�
�ʴ�Ϊ��ƽ��ѹǿ��ʹ©����Һ��˳�����£�ˮԡ��
��3�������״��ֲ�Ʒ�к������ѡ��屽���������������л�������״��ķе���ߣ�����������������ķ�����ȥ�л����ʣ�
���ڼ�ʽ�廯þ����ˮ���������л��ܼ�������ϴ��Һѡ��ˮ����ѡa��
���ϴ���Ƿ�ɾ���һ�㲽���ǣ�ȡ�������һ��ϴ��Һ���Թ��У��μ���������Һ�����������ɣ�����ϴ�Ӹɾ�����֮��δϴ�Ӹɾ���
�ʴ�Ϊ����������a��ȡ�������һ��ϴ��Һ���Թ��У��μ���������Һ�����������ɣ�����ϴ�Ӹɾ�����֮��δϴ�Ӹɾ���
��4����2-OH��H2����֪�����״������ʵ�����$\frac{0.1008L}{22.4L/mol}$��2=0.009mol�����Բ�Ʒ�������״���������0.009mol��260g/mol=2.34g�����Ʒ�������״�����������=$\frac{2.34g}{2.60g}$��100%=90%��
�ʴ�Ϊ��90%��
���� ���⿼���л���ϳ�ʵ�顢���ʵķ�����ϴ�ӵȻ�����������ʵ��װ�õķ������۵ȣ��ϺõĿ���ѧ�������ݵ�Ӧ�á��Ķ���ȡ��Ϣ�������Լ�֪ʶǨ��Ӧ�ã��Ѷ��еȣ�

 ��У����ϵ�д�
��У����ϵ�д������ǵؿ��ﺬ�����Ľ���Ԫ�آڵ���������Ŀǰ���ڱ��к�Ԫ����������
��Na�Ƕ�����Ԫ����ԭ�Ӱ뾶��������Ԫ�� ��F�Ƿǽ�����ǿ��Ԫ�أ�
| A�� | ȫ�� | B�� | ������ȫ�� | C�� | ������ȫ�� | D�� | ������ȫ�� |
| A�� | ��ƽ����ϵ�м�������KCl�����Fe��SCN��3���壬��Һ��ɫ����dz | |
| B�� | ��ƽ����ϵ�м�������FeCl3�����KSCN���壬��Һ��ɫ���� | |
| C�� | ��ƽ����ϵ�м���ˮϡ�ͣ�ƽ�ⲻ�ƶ�������Һ��ɫ��dz | |
| D�� | �÷�Ӧ�����ӷ���ʽΪ��3KSCN+Fe3+�TFe��SCN��3��Ѫ��ɫ��+3K+ |
| A�� | Cl2+2I-�T2Cl-+I2 | B�� | �ȶ��ԣ�HCl��HI | ||
| C�� | ���� HClO4��HIO4 | D�� | ���� HClO3��HIO3 |

��֪����������Cl2��Ӧ����PCl3�������Cl2��Ӧ����PCl5��PCl3��ˮ��ǿ��ˮ������H3PO3��HCl����O2������POCl3��POCl3����PCl3��PCl3��POCl3���۷е������
| ���� | �۵�/�� | �е�/�� |
| PCl3 | -112 | 75.5 |
| POCl3 | 2 | 105.3 |
��1��B����װ�Լ���ŨH2SO4��E����ˮ������������PCl3��ֹ��ӷ���
��2��F�м�ʯ�ҵ����������ն������������ֹ�����е�ˮ����������ƿ�к�PCl3 ��Ӧ��
��3��ʵ��ʱ�����װ�������Ժ��ȴ�K3ͨ������CO2����Ѹ�ټ�����ף�ͨ����CO2���������ž�װ���еĿ�������ֹ������ȼ��
��4���ֲ�Ʒ�г�����POCl3��PCl5�ȣ���������ȳ�ȥPCl5��ͨ��������ʵ��������ƣ������ɵõ��ϴ�����PCl3��
��5��ʵ�����ʱ����������C�е��Լ����ն����������C�з�Ӧ�����ӷ���ʽΪCl2+2OH-=Cl-+ClO-+2H2O��
��6��ͨ�����淽���ɲⶨ��Ʒ��PCl3������������
��Ѹ�ٳ�ȡ1.00g��Ʒ����ˮ��Ӧ�����250mL��Һ��
��ȡ������Һ25.00mL�������м���10.00mL 0.1000mol/L��ˮ����ַ�Ӧ��
�����������Һ�м��뼸�ε�����Һ����0.1000mol/L��Na2S2O3����Һ�ζ���
���ظ��ڡ��۲�����ƽ������Na2S2O3��Һ8.40mL��
��֪��H3PO3+I2�TH3PO4+2HI��I2+2Na2S2O3�T2NaI+Na2S4O6��
�����������ݣ�����ⶨ������û��������Ӧ���ò�Ʒ��PCl3����������Ϊ79.75%��

��1����֪����ٷ�Ӧ�������ķ�ӦΪ��6I2+11KClO3+3H2O�T6KH��IO3��2+5KCl+3Cl2�����÷�Ӧ�Ļ�ԭ����ΪKCl��Cl2��
��2�������±�����ص��ܽ�ȣ������۵õ�����ؾ��壬�㽨��ķ����ǽ��½ᾧ��
| �¶�/�� | 20 | 40 | 60 | 80 |
| KIO3g/100gˮ | 8.08 | 12.6 | 18.3 | 244.8 |
�ⶨ�ӵ�ʳ���е�ĺ�����ѧ������Ƶ�ʵ�鲽�����£�
a��ȷ��ȡwgʳ�Σ�����������ˮʹ����ȫ�ܽ⣻
b����ϡ�����ữ������Һ���������KI��Һ��ʹKIO3��KI��Ӧ��ȫ��
c���Ե���Ϊָʾ������μ������ʵ���Ũ��Ϊ2.00��10-3mol•L-1��Na2S2O3��Һ10.0mL��ǡ�÷�Ӧ��ȫ����ӵ�ʳ����Ʒ�еĵ�Ԫ�غ�����$\frac{0.01}{3w}$mol•kg-1���Ժ�w�Ĵ���ʽ��ʾ����
��ѧ�����ֽ���������ʵ�飺
| �������� | ʵ������ |
| ȡ1g������NaCl����3mLˮ������Һ | ��Һ�ޱ仯 |
| ����5�ε�����Һ��1mL0.1mol��L-1KI��Һ���� | ��Һ�ޱ仯 |
| Ȼ���ٵ���1��1mol��L-1��H2SO4���ӷ��� | ��Һ����ɫ |
����ѧ���ҵ�ʵ���������ѧ����ʵ����������Ҫ���ۣ�ƫ������I-�ᱻ�����е�O2����ΪI2��
��4��ijѧϰС��Լӵ��ν�������ʵ�飺ȡһ����ij�ӵ��Σ����ܺ���KIO3��KI��Mg2+��Fe3+��������������ˮ�ܽ⣬����ϡ�����ữ����������Һ��Ϊ3�ݣ���һ����Һ�еμ�KSCN��Һ���Ժ�ɫ���ڶ�����Һ�м�����KI���壬��Һ�Ե���ɫ����CCl4��ȡ���²���Һ���Ϻ�ɫ����������Һ�м�������KIO3����μӵ����Լ�����Һ����ɫ��
�ٸ�������ʵ�����ӵ����п��ܺ��е������в���ȷ������Mg2+��
�ڵڶ�����Һ�м�������KI�����Ӧ�����ӷ���ʽΪ2Fe3++2I-=2Fe2++I2��IO3-+5I-+6H+�T3I2+3H2O��
| A�� | ��������ˮ | B�� | ���� | C�� | ����NaHSO4���� | D�� | ����Ũ���� |
| A�� | ij���ʵ���Һ����ˮ�������c��H+��=1��10-amol/L����a��7ʱ�������Һ��pHһ��Ϊ14-a | |
| B�� | ij��Һ�д��ڵ�������S2-��HS-��OH-��Na+��H+��������Ũ��һ����c��Na+����c��S2-����c��OH-����c��HS-����c��H+�� | |
| C�� | ��0.2 mol/L��ijһԪ��HA��Һ��0.1mol/L NaOH��Һ�������Ϻ���ҺpH����7����Ӧ��Ļ��Һ��2c��OH-��=2c��H+��+c��HA��-c��A-�� | |
| D�� | ��Na2CO3��Һ��CH3COONa��Һ��NaOH��Һ����3����ҺpH��Ϊ9�����������ʵ���Ũ�ȴ�С˳���Ǣ٣��ڣ��� |
