��Ŀ����
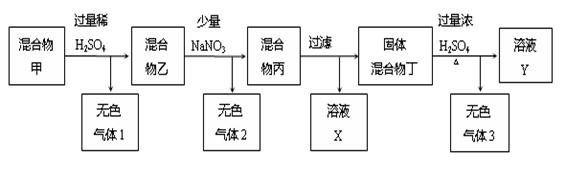
�ס��ҡ���������X���ɶ�����Ԫ����ɵĴ��������XΪ���ʡ���������ת���� ϵ����ͼ��ʾ��ijЩ������ȥ����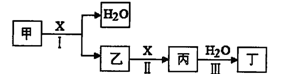
��ش��������⣺
��1��������һԪǿ�ᣬ�ס����ǹ�ҵ����������Ҫ;����
��д����ӦI�Ļ�ѧ����ʽ�� ��
�ڳ����£�1 mol��������ӦIII�ų�46kJ�������÷�Ӧ���Ȼ�ѧ����ʽΪ�� ��
���ڳ����£���V1L pH= 11�ļ���Һ�м���V2L pH=3�����ᣬ����Ӧ����Һ��pH<7����V1��V2�Ĺ�ϵΪV1 V2��ѡ���������������������������Һ�и������ӵ�Ũ���ɴ�С��˳������ǣ� ��дһ�ּ��ɣ���
��2�������Ƕ�Ԫ���ᣬ�����������塣
���ݻ�Ϊ2L���ݻ��̶����ܱ������У�����(g)��H2O(g)���±������ֱ���з�Ӧ����(g)+H2O(g)  ��(g) + H2(g)���õ��������ݣ�
��(g) + H2(g)���õ��������ݣ�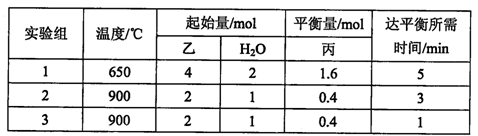
�ٸ÷�Ӧ������ӦΪ ������ȡ����ȡ�����Ӧ��
��900��ʱ��������Ӧ��������ʼ���ֱ�����������
���ʱ��Ӧ��v������ v���棩�����������������������
��ʵ��3��ʵ��2��ȣ��ı������������ ��
��15�֣���1����4NH3+5O2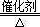 4NO+6H2O��3�֣�
4NO+6H2O��3�֣�
��3NO2(g)+H2O(l)==2HNO3(aq)+NO(g) ��H=��138kJmol��1��3�֣�
��<��1�֣� c(Cl��)>c(NH4+)>c(H+)>c(OH��)��c(Cl��)>c(H+)>c(NH4+)>c(OH��)��2�֣�
��2���ٷ��ȣ�2�֣� ��<��2�֣� ��ʹ���˴�����2�֣�
���������������1��������֪��Ϣ��֪���ס��ҡ���������X�ֱ���NH3��NO��NO2��HNO3��O2����I�ķ�ӦʽΪ4NH3+5O2 4NO+6H2O�����������֪��NO2(g)+1/3H2O(l)=2/3HNO3(aq)+1/3NO(g) ��H=��46kJ/mol����3NO2(g)+H2O(l)==2HNO3(aq)+NO(g) ��H=��138kJmol��1������V1=V2����c(NH3?H2O)>c(HCl)����c?V��֪�������������Һ�ʼ��ԣ���V1<V2�������Һ�ſ��������ԣ�����������̶Ƚ�С�������Ի����Һ��c(Cl��)>c(NH4+)>c(H+)>c(OH��)������������̶Ƚϴ���c(Cl��)>c(H+)>c(NH4+)>c(OH��)����2��������֪��Ϣ��֪���ס��ҡ���������X�ֱ���C��CH4��CO��CO2��H2CO3��O2����
4NO+6H2O�����������֪��NO2(g)+1/3H2O(l)=2/3HNO3(aq)+1/3NO(g) ��H=��46kJ/mol����3NO2(g)+H2O(l)==2HNO3(aq)+NO(g) ��H=��138kJmol��1������V1=V2����c(NH3?H2O)>c(HCl)����c?V��֪�������������Һ�ʼ��ԣ���V1<V2�������Һ�ſ��������ԣ�����������̶Ƚ�С�������Ի����Һ��c(Cl��)>c(NH4+)>c(H+)>c(OH��)������������̶Ƚϴ���c(Cl��)>c(H+)>c(NH4+)>c(OH��)����2��������֪��Ϣ��֪���ס��ҡ���������X�ֱ���C��CH4��CO��CO2��H2CO3��O2����
CO(g) + H2O(g)  CO2(g) + H2(g)
CO2(g) + H2(g)
����ֵ���ʼŨ��/mol?L��1 2 1 0 0
����ֵı仯Ũ��/mol?L��1 0.8 0.8 0.8 0.8
����ֵ�ƽ��Ũ��/mol?L��1 1.2 0.2 0.8 0.8
650��ʱ��K=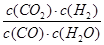 =
=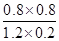 =8/3
=8/3
CO(g) + H2O(g)  CO2(g) + H2(g)
CO2(g) + H2(g)
����ֵ���ʼŨ��/mol?L��1 1 0.5 0 0
����ֵı仯Ũ��/mol?L��1 0.2 0.2 0.2 0.2
����ֵ�ƽ��Ũ��/mol?L��1 0.8 0.3 0.2 0.2
900��ʱ��K=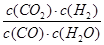 =
= =1/6
=1/6
650���900��ʱ��K��С���������¶ȣ�ƽ�����淴Ӧ�����ƶ�����������Ӧ�Ƿ��ȷ�Ӧ��
��900��ʱ��Q=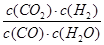 =
=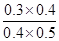 =3/5>1/6��˵���÷�ӦӦ���淴Ӧ�����ƶ������ܴﵽ���¶��µĻ�ѧƽ�⣬��v(��)>v(��)��ʵ��2��3��ƽ��û���ƶ�������Ӧ���ʼӿ죬˵��2��3����ʹ���˴�����
=3/5>1/6��˵���÷�ӦӦ���淴Ӧ�����ƶ������ܴﵽ���¶��µĻ�ѧƽ�⣬��v(��)>v(��)��ʵ��2��3��ƽ��û���ƶ�������Ӧ���ʼӿ죬˵��2��3����ʹ���˴�����
���㣺��������Ĺ�ҵ�Ʒ������Ĵ��������Ȼ�ѧ����ʽ����Һ������ԡ��������Һ������Ũ�ȴ�С��ϵ����ѧƽ�ⳣ�����¶ȶԻ�ѧƽ���Ӱ�졢��ѧ��Ӧ���еķ���Ӱ�컯ѧ��Ӧ���ʺͻ�ѧƽ������ص����֪ʶ��

ϡ��Ԫ�������ڱ��Т� B���֡��ƺ���ϵԪ�ص��ܳƣ����Ƕ��Ǻܻ��õĽ��������ʼ�Ϊ���ƣ��������ϼ�Ϊ+3�������ƣ�Y��Ԫ���Ǽ���ͳ�������Ҫ���ϡ�
�ҹ��̲��ŷḻ���ƿ�ʯ�� Y2 FeBe2Si2O10�����Դ˿�ʯΪԭ�����������ƣ�Y2O3������Ҫ�������£�
��֪��
I���йؽ��������γ������������ʱ��pH���±���
| | ��ʼ����ʱ��pH | ��ȫ����ʱ��pH |
| Fe3+ | 2��7 | 3��7 |
| Y3+ | 6��0 | 8��2 |
�������ڱ��У��롢��Ԫ�ش��ڵڶ����ں͵������ڵĶԽ���λ�ã���ѧ�������ơ�
��1���ƿ�ʯ��Y2 FeBe2Si2O10������������������ʽ�ɱ�ʾΪ ��
��2������Na2 SiO3��Na2BeO2�Ļ����Һ���Ƶ�Be(OH)2��������
�����ѡ�����ᡢ ������ĸ���ţ������Լ�����ͨ����Ҫ��ʵ���������ʵ�֡�
a��NaOH��Һ b����ˮ c��CO2�� d��HNO3
��д��Na2BeO2���������ᷢ����Ӧ�����ӷ���ʽ�� ����Ҫ��ʵ�����Ӧ�� ��
��3��ΪʹFe3+������ȫ�����ð�ˮ����pH =a����aӦ������ �ķ�Χ�ڣ������Ӱ�
ˮ����pH =b������Ӧ�����ӷ���ʽΪ ����Һ��Fe3+��ȫ�������ж����� ��
��4��д��������[Y2(C2O4)3��Nh2O]���յĻ�ѧ����ʽ ��
ͬһ���������Ԫ�ص�ԭ������֮����ܵ���
| A��16 | B��26 | C��36 | D��46 |
���и���Ϊ���ڱ���һ���֣�����Ϊԭ����������������ȷ���ǣ� ��
| | 2 | | | 2 | 3 | 4 | | | 6 | | | | 6 | 7 |
| 11 | | | | 11 | | 11 | 12 | 13 | | 14 | | |||
| 19 | | | | 19 | | | 24 | | 31 | 32 | |
 ��X�е�һ�֡�
��X�е�һ�֡� C��CH3COO D��SiO
C��CH3COO D��SiO