��Ŀ����
����A��B��C��D��E��F���ֶ�����Ԫ�أ��ڻ�ѧ��Ӧ�о����γɼ������ӻ������ӣ���A��B��C��D���Ӿ�����ͬ�ĵ��Ӳ�ṹ����֪��
�ٳ����£�F�ĵ�����һ����ɫ���壬������ɱ����������
��A���������������NaOH��Һ�������������
��C���⻯�����G�Ǿ���10���ӵ������ҿ��Է�������ת����G P
P Q
Q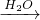 M+P
M+P
��E��D��ͬ����Ԫ�أ��⻯�H2E��������Ϊ��̬��
��B��D���γ���������Ϊ1��1��2��1�����ӻ�����X��Y��
��ش��������⣺
��1��AԪ�ص�ԭ�ӽṹʾ��ͼ��______��B2E�ĵ���ʽΪ______��
��2��������X������ѧ��������______��
��3��G����ļ��鷽��Ϊ______��
��4��д��F������E���⻯�ﷴӦ�ķ���ʽ����������ת�Ʒ������Ŀ______��
�⣺A��B��C��D��E��F���ֶ�����Ԫ�أ��ڻ�ѧ��Ӧ�о����γɼ������ӻ������ӣ���A��B��C��D���Ӿ�����ͬ�ĵ��Ӳ�ṹ��
�ٳ����£�F�ĵ�����һ����ɫ���壬������ɱ�����������ƶ�ΪCl2��
��A���������������NaOH��Һ�������������ᣬ˵���������������ж�ΪAl2O3��AΪAlԪ�أ�
��C���⻯�����G�Ǿ���10���ӵ������ڻ�ѧ��Ӧ�о����γɼ������ӻ������ӣ���A��B��C��D���Ӿ�����ͬ�ĵ��Ӳ�ṹ�����Է�������ת����G P
P Q
Q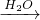 M+P
M+P
�ƶ�CΪNԪ�أ�GΪNH3��DΪOԪ��PΪNO��QΪNO2��MΪHNO3��
��E��D��ͬ����Ԫ�أ��⻯�H2E��������Ϊ��̬���ж�EΪS��
��B��DΪO���γ���������Ϊ1��1��2��1�����ӻ�����X��Y�����A��B��C��D���Ӿ�����ͬ�ĵ��Ӳ�ṹ���ж�BΪNa��XΪNa2O2��YΪNa2O��
��1��AԪ��ΪAl��ԭ�ӽṹʾ��ͼ�ǣ�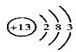 ��B2EΪNa2S�����������ӻ�������Ƶĵ���ʽΪ��
��B2EΪNa2S�����������ӻ�������Ƶĵ���ʽΪ��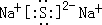 ��
��
�ʴ�Ϊ��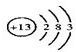 ��
��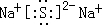 ��
��
��2��������XΪNa2O2��������ѧ�������У����Ӽ����Ǽ��Թ��ۼ����ʴ�Ϊ�����Ӽ����Ǽ��Թ��ۼ���
��3��G����ΪNH3���鷽��Ϊ����ʪ��ĺ�ɫʯ����ֽ�������壬����ֽ����֤������Ϊ������
�ʴ�Ϊ����ʪ��ĺ�ɫʯ����ֽ�������壬����ֽ����֤������Ϊ������
��4��F����ΪCl2��E���⻯��ΪH2S��Ӧ�ķ���ʽΪ��H2S+Cl2=S+2HCl����������ת�Ʒ������Ŀ�Ļ�ѧ����ʽΪ��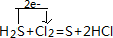 ��
��
�ʴ�Ϊ�� ��
��
�������ٳ����£�F�ĵ�����һ����ɫ���壬������ɱ�����������ƶ�ΪCl2��
��A���������������NaOH��Һ�������������ᣬ˵���������������ж�ΪAl2O3��
��C���⻯�����G�Ǿ���10���ӵ������ڻ�ѧ��Ӧ�о����γɼ������ӻ������ӣ���A��B��C��D���Ӿ�����ͬ�ĵ��Ӳ�ṹ�����Է�������ת����G P
P Q
Q M+P
M+P
�ƶ�CΪNԪ�أ�GΪNH3��DΪOԪ��PΪNO��QΪNO2��MΪHNO3��
��E��D��ͬ����Ԫ�أ��⻯�H2E��������Ϊ��̬���ж�EΪS��
��B��DΪO���γ���������Ϊ1��1��2��1�����ӻ�����X��Y�����A��B��C��D���Ӿ�����ͬ�ĵ��Ӳ�ṹ���ж�BΪNa��XΪNa2O2��YΪNa2O�������жϳʵ����ʻش�
���������⿼��������ת����ϵ������ԭ�ӽṹ���ж�Ӧ�ã���Ҫ��ԭ�ӽṹ�ķ���������Ԫ���жϣ��ṹ�����ʵķ������⣬���ջ����ǽ���ؼ�����Ŀ�Ѷ��еȣ�
�ٳ����£�F�ĵ�����һ����ɫ���壬������ɱ�����������ƶ�ΪCl2��
��A���������������NaOH��Һ�������������ᣬ˵���������������ж�ΪAl2O3��AΪAlԪ�أ�
��C���⻯�����G�Ǿ���10���ӵ������ڻ�ѧ��Ӧ�о����γɼ������ӻ������ӣ���A��B��C��D���Ӿ�����ͬ�ĵ��Ӳ�ṹ�����Է�������ת����G
 P
P Q
Q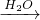 M+P
M+P�ƶ�CΪNԪ�أ�GΪNH3��DΪOԪ��PΪNO��QΪNO2��MΪHNO3��
��E��D��ͬ����Ԫ�أ��⻯�H2E��������Ϊ��̬���ж�EΪS��
��B��DΪO���γ���������Ϊ1��1��2��1�����ӻ�����X��Y�����A��B��C��D���Ӿ�����ͬ�ĵ��Ӳ�ṹ���ж�BΪNa��XΪNa2O2��YΪNa2O��
��1��AԪ��ΪAl��ԭ�ӽṹʾ��ͼ�ǣ�
 ��B2EΪNa2S�����������ӻ�������Ƶĵ���ʽΪ��
��B2EΪNa2S�����������ӻ�������Ƶĵ���ʽΪ��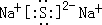 ��
���ʴ�Ϊ��
 ��
��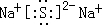 ��
����2��������XΪNa2O2��������ѧ�������У����Ӽ����Ǽ��Թ��ۼ����ʴ�Ϊ�����Ӽ����Ǽ��Թ��ۼ���
��3��G����ΪNH3���鷽��Ϊ����ʪ��ĺ�ɫʯ����ֽ�������壬����ֽ����֤������Ϊ������
�ʴ�Ϊ����ʪ��ĺ�ɫʯ����ֽ�������壬����ֽ����֤������Ϊ������
��4��F����ΪCl2��E���⻯��ΪH2S��Ӧ�ķ���ʽΪ��H2S+Cl2=S+2HCl����������ת�Ʒ������Ŀ�Ļ�ѧ����ʽΪ��
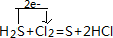 ��
���ʴ�Ϊ��
 ��
���������ٳ����£�F�ĵ�����һ����ɫ���壬������ɱ�����������ƶ�ΪCl2��
��A���������������NaOH��Һ�������������ᣬ˵���������������ж�ΪAl2O3��
��C���⻯�����G�Ǿ���10���ӵ������ڻ�ѧ��Ӧ�о����γɼ������ӻ������ӣ���A��B��C��D���Ӿ�����ͬ�ĵ��Ӳ�ṹ�����Է�������ת����G
 P
P Q
Q M+P
M+P�ƶ�CΪNԪ�أ�GΪNH3��DΪOԪ��PΪNO��QΪNO2��MΪHNO3��
��E��D��ͬ����Ԫ�أ��⻯�H2E��������Ϊ��̬���ж�EΪS��
��B��DΪO���γ���������Ϊ1��1��2��1�����ӻ�����X��Y�����A��B��C��D���Ӿ�����ͬ�ĵ��Ӳ�ṹ���ж�BΪNa��XΪNa2O2��YΪNa2O�������жϳʵ����ʻش�
���������⿼��������ת����ϵ������ԭ�ӽṹ���ж�Ӧ�ã���Ҫ��ԭ�ӽṹ�ķ���������Ԫ���жϣ��ṹ�����ʵķ������⣬���ջ����ǽ���ؼ�����Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
 Сѧ��ʱ��ѵϵ�д�
Сѧ��ʱ��ѵϵ�д�
�����Ŀ
 ����A��B��C��D��E��F���ֶ�����Ԫ�أ����ǵ�ԭ��������������D��E���⻯����ӹ��Ͷ���V�ͣ�A��B������������֮����C��������������ȣ�A�ֱܷ���B��C��D�γɵ���������ȵķ��ӣ���A��D���γɵĻ���������¾�ΪҺ̬��
����A��B��C��D��E��F���ֶ�����Ԫ�أ����ǵ�ԭ��������������D��E���⻯����ӹ��Ͷ���V�ͣ�A��B������������֮����C��������������ȣ�A�ֱܷ���B��C��D�γɵ���������ȵķ��ӣ���A��D���γɵĻ���������¾�ΪҺ̬��






