��Ŀ����
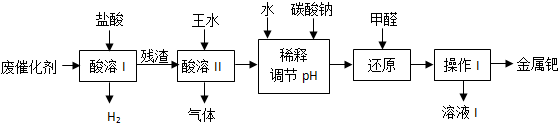
17��п������BaSO4��ZnS�Ļ���ZnS������ˮ��ij���������ؾ�ʯΪԭ���Ʊ�п���ף����������л����������������Ҫ�ɷ�ΪCO2��CO������SO2���������ȣ���Ϊ��ֹ��Ⱦ���������ԭ�������ʣ������в��������������̣�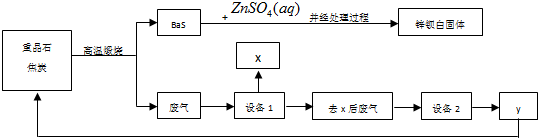
������������̻ش��������⣺
��1���ؾ�ʯ�Ļ�ѧʽΪBaSO4��п�������ڵ��ư�ɫ��dzɫ���ᣮ
��2���������̣�a���IJ���Ϊ���ٹ��ˣ���ϴ�ӣ��۸��
��3���豸1����ȴ�����������豸1ǰ��ĵ����ܹ�������խ������ʹ�����ܶ�������ԭ������������ȴ����˹��壮
��4������п�����к���S2-�ķ�����ȡ��Ʒ�������ᣬ��������������ʹʪ�Ĵ���Ǧ��ֽ��ڣ�˵���������ӣ�
��5���豸2�����õ�ϴ�Ӽ���NaOH��Һ����y�Ļ�ѧʽΪCO��д���豸2�з�����Ӧ�����ӷ���ʽ��SO2+2OH-��SO32-+H2O��CO2+2OH-��CO32-+H2O��
��6�����ýᾧ�������豸2��Һ�е����Σ����з���ǰ����Ҫ��õ��Ǹ����ʵ��ܽ�����¶ȵı仯�����
��7���Ʊ������еõ�����ǣ����˿������������⣬��������;Ϊ������ҩ��ũҩ�Լ���Ƥ�����ȣ�����дһ�֣���
���� �������еĹ�ҵ���̿�֪���ؾ�ʯ��̼����������ԭ��Ӧ���ؾ�ʯ����ԭ��������������������п�����÷ֽⷴӦ�ɵ����ᱵ����п��п���ף���������Ҫ�ɷ�ΪCO2��CO������SO2���������ȣ������豸1��X���豸1����ȴ������������XӦΪS�����豸1ǰ��ĵ����ܹ�������խ����������ȴ����˹��壬��ʹ�����ܶ�����ȥ���ķ�����Ҫ��CO2��CO������SO2�������豸2���豸2�����õ�ϴ�Ӽ���NaOH��Һ��CO2��SO2�������������գ���õ���YΪCO��CO��ѭ�����ã���ԭ�ؾ�ʯ���ݴ˴��⣮
��� �⣺�������еĹ�ҵ���̿�֪���ؾ�ʯ��̼����������ԭ��Ӧ���ؾ�ʯ����ԭ��������������������п�����÷ֽⷴӦ�ɵ����ᱵ����п��п���ף���������Ҫ�ɷ�ΪCO2��CO������SO2���������ȣ������豸1��X���豸1����ȴ������������XӦΪS�����豸1ǰ��ĵ����ܹ�������խ����������ȴ����˹��壬��ʹ�����ܶ�����ȥ���ķ�����Ҫ��CO2��CO������SO2�������豸2���豸2�����õ�ϴ�Ӽ���NaOH��Һ��CO2��SO2�������������գ���õ���YΪCO��CO��ѭ�����ã���ԭ�ؾ�ʯ��
��1�����ᱵ���������ؾ�ʯ�������ؾ�ʯ�Ļ�ѧʽΪBaSO4�����ᱵ����п���ǰ�ɫ���壬����п�������ڵ��ư�ɫ��dzɫ���ᣬ
�ʴ�Ϊ��BaSO4���ף�
��2�����������ǽ���������п�����÷ֽⷴӦ�ɵ����ᱵ����п�����õ����壬����ʵ�鲽��Ϊ���ٹ��ˣ���ϴ�ӣ��۸��
�ʴ�Ϊ�����ˣ����
��3����������ķ�����֪��ԭ������������ȴ����˹��壬
�ʴ�Ϊ����������ȴ����˹��壻
��4������S2-�����ʿ�֪������п�����к���S2-�ķ�����ȡ��Ʒ�������ᣬ��������������ʹʪ�Ĵ���Ǧ��ֽ��ڣ�˵���������ӣ�
�ʴ�Ϊ��ȡ��Ʒ�������ᣬ��������������ʹʪ�Ĵ���Ǧ��ֽ��ڣ�˵���������ӣ�
��5��CO2��SO2�������������գ���y�Ļ�ѧʽΪCO���豸2�з�����Ӧ�����ӷ���ʽ��SO2+2OH-��SO32-+H2O��CO2+2OH-��CO32-+H2O��
�ʴ�Ϊ��CO��SO2+2OH-��SO32-+H2O��CO2+2OH-��CO32-+H2O��
��6���豸2��Һ�е�����Ϊ̼���ơ��������Ƶȣ�����������ˮ�ģ��ɸ������ǵ��ܽ�����¶ȵĹ�ϵ���ýᾧ��������з���ǰ��������Ҫ��õ��Ǹ����ʵ��ܽ�����¶ȵı仯�����
�ʴ�Ϊ���ᾧ�������ʵ��ܽ�����¶ȵı仯�����
��7�����������;��֪���Ʊ������еõ�����ǣ����˿������������⣬����������������ҩ��ũҩ�Լ���Ƥ�����ȣ�
�ʴ�Ϊ��������ҩ��ũҩ�Լ���Ƥ�����ȣ�
���� ���⿼���������Ʊ����̷����жϡ��������������ʳɷַ��������ʵķ����ᴿ�����ӷ���ʽ����д�ȣ���Ŀ�Ѷ��еȣ�ע��Ԫ�ػ���֪ʶ�빤ҵ�������л���ϣ�

 �ľ�ͼ���ʱ�ȷ�ϵ�д�
�ľ�ͼ���ʱ�ȷ�ϵ�д�
| A�� | ȼ�ϵ���ܵ����ӷ�Ӧ����ʽΪ��2Al+3HO2-�T2AlO2-+2H2O | |
| B�� | ���ʱ��Al����2.7g����������������Ϊ1.12L | |
| C�� | �缫b�Ǹ������ҷ�Ӧ��õ缫��pH���� | |
| D�� | �������У����ӵ�������a��d��c��b�� |
A��ͬλ�� B��ͬ���칹�� C��ͬϵ�� D��ͬ�������� E��ͬһ���� F��ͬ������
| �������� | ���ϵ | �������� | ���ϵ |
| ���ȷ������ȼ��� | ��뭡�뮡�� | ||
| ��һ�ȼ��������Ȼ�̼ | �ݰ�������� | ||
| �������������� | �����������춡�� |
| A�� | �ﵽƽ�������ѹǿ�����ڸ÷�Ӧƽ�������ƶ� | |
| B�� | �ﵽ��ѧƽ��ʱ��v����A2��=2v����B2�� | |
| C�� | �����¶ȣ�����Ӧ�������ӣ�����Ӧ���ʼ�С | |
| D�� | �ﵽƽ������¶�A2�������ת���ʼ�С |
| ��� | ���ʣ����ʣ� | �����Լ� | ���뷽�� |
| A | �屽��Br2�� | �� | ��ȡ����Һ |
| B | �������������ᣩ | ����̼������Һ | ��Һ |
| C | CH4��C2H4�� | ���Ը������ | ϴ�� |
| D | �����������ᣩ | ����������Һ | ���� |
| A�� | A | B�� | B | C�� | C | D�� | D |
| A�� | ��״���£�11.2 L���ȼ��飨 CH2Cl2�������ķ�����Ϊ0.5NA | |
| B�� | �ܱ�������2molNO��1molO2��ַ�Ӧ������ķ�����Ϊ2NA | |
| C�� | 1mol�ǻ���-OH�������ĵ�������Ϊ10NA | |
| D�� | 10g��������Ϊ46%���Ҵ���Һ�У���ԭ�ӵ�����Ϊ1.2NA |
| A�� | Cu2+��SO42- | B�� | K+��SO42- | C�� | Cu2+��Cl- | D�� | Ag+��NO3- |