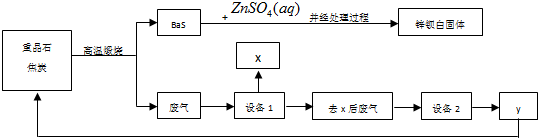
��Ŀ����
5���٣�Pd���������벬���ƣ���ҵ�ϴӷϴ�������Ҫ�ɷ����ٺͻ���̿����������������п���л����٣������������̣�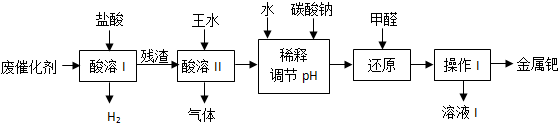
��ش��������⣺
��1������I��Ŀ���dz�ȥ����п�����ʣ�
��2�������ܢ�ʱ��������ˮ�ڼ���������������Ҫ��Ӧ�ǣ�3Pd+12HCl+2HNO3$\frac{\underline{\;\;��\;\;}}{\;}$3H2PdCl4+2NO��+4H2O
д����������һ��Ҫ�ɷ���Ũ���ᷴӦ�Ļ�ѧ����ʽ��C+4HNO3��Ũ��$\frac{\underline{\;\;��\;\;}}{\;}$CO2��+4NO2��+2H2O��
�����ܢ�������¶Ȳ��˹��ߣ����˿���һ����Ӧ�����⣬��ԭ����ܻ��з�ֹ����ֽ⣬��ֹ���ᡢ����ӷ���
�������������ռ��������壬��д�����պ���Һ�к��е����ʵĻ�ѧʽ��NaOH��NaNO2��NaNO3��Na2CO3��
��3��ʹ�ü�ȩ��ԭ�ٵĻ�����ʱ����Һ�뱣�ּ��ԣ��������ɼ�ȩ�Ķ�����ģ�ԭ�������������£���ȩ�ᱻ����������
��4������I�������ǹ��ˣ���ҺI���ܺ��е��л�����ΪHCOO-��
��5������������ڽ�������ǰ����Ƚ��ϴ�����700���½������գ�ͬʱ����ͨ���������Ŀ���dz�ȥ�ϴ����еĻ���̿��������ˮ�����ģ�
���� �ϴ�������Ҫ�ɷ����ٺͻ���̿����������������п�����ϴ������ܵõ�����������п�����ᷴӦ�����˵õ�������Ҫ�ɷ�Ϊ�ٺͻ���̿��������ˮ������ˮ�ڼ�������������Ӧ����һ���������壬��ҺΪH2PdCl4������ˮ��̼����ϡ�͵�����ҺPH�������ȩ��ԭ�ٵĻ�����õ������ٹ��˵õ���Һ���к��к��м�ȩ�������õ��ļ����Σ�
��1������ʵ���Ŀ���Լ��ϴ�������Ҫ�ɷ����ٺͻ���̿����������������п��
��2���ٴ�������һ��Ҫ�ɷ��ǻ���̼��
�����ܢ�ʹ�õ�����ˮ����ˮ����������ֽ⣬���ᡢ�����ӷ���
���ռ�����һ�������������Ļ�����壬�����������ơ��������ƣ�
��3��������Һ��������������Ի�������ȩ��
��4���������̿�֪�����Ƿ���������Һ�ǹ��ˣ���ȩ�����������ɼ��ᣬ�ڼ���Һ�����ɼ����Σ�
��5�����ݷϴ����еĺ��л���̿������ͨ��������ܳ�ȥ����̿��ͬʱ������ˮ�����ģ�
��� �⣺�ϴ�������Ҫ�ɷ����ٺͻ���̿����������������п�����ϴ������ܵõ�����������п�����ᷴӦ�����˵õ�������Ҫ�ɷ�Ϊ�ٺͻ���̿��������ˮ������ˮ�ڼ�������������Ӧ����һ���������壬��ҺΪH2PdCl4������ˮ��̼����ϡ�͵�����ҺPH�������ȩ��ԭ�ٵĻ�����õ������ٹ��˵õ���Һ���к��к��м�ȩ�������õ��ļ����Σ�
��1���ϴ�������Ҫ�ɷ����ٺͻ���̿����������������п����ʵ���Ŀ���Ǵӷϴ�������ȡ�٣���������I��Ŀ���dz�ȥ����п�����ʣ�
�ʴ�Ϊ����ȥ����п�����ʣ�
��2���ٴ�������һ��Ҫ�ɷ��ǻ���̼��̼��Ũ���ᷴӦ�Ļ�ѧ����ʽΪC+4HNO3��Ũ��$\frac{\underline{\;\;��\;\;}}{\;}$ CO2��+4NO2��+2H2O��
�ʴ�Ϊ��C+4HNO3��Ũ��$\frac{\underline{\;\;��\;\;}}{\;}$ CO2��+4NO2��+2H2O��
�����ܢ�������¶Ȳ��˹��ߣ����˿���һ����Ӧ�����⣬���ܢ�ʹ�õ�����ˮ����ˮ����������ֽ⣬���ᡢ�����ӷ���
�ʴ�Ϊ����ֹ����ֽ⣬��ֹ���ᡢ����ӷ���
�����������ռ����ջ��п�����������ռ�����һ�������������Ļ�����壬�����������ơ��������ƣ����պ���Һ�к��е����ʵĻ�ѧʽ��NaOH��NaNO2��NaNO3��Na2CO3��
�ʴ�Ϊ��NaNO3��Na2CO3��
��3��ʹ�ü�ȩ��ԭ�ٵĻ�����ʱ����Һ�뱣�ּ��ԣ�����ˮ��Ӧ��ʣ�����ᣬ������Һ��������������Ի�������ȩ��
�ʴ�Ϊ�����������£���ȩ�ᱻ����������
��4���������̿�֪�����Ƿ���������Һ�ǹ��ˣ���ȩ�����������ɼ��ᣬ�ڼ���Һ�����ɼ����Σ���ҺI���ܺ��е��л�����ΪHCOO-��
�ʴ�Ϊ�����ˣ�HCOO-��
��5���ϴ����еĺ��л���̿������ͨ��������ܳ�ȥ����̿��ͬʱ������ˮ�����ģ�
�ʴ�Ϊ����ȥ�ϴ����еĻ���̿��������ˮ�����ģ�
���� �����Դӷϴ�������ȡ��Ϊ�����������˹�ҵ���̵ķ��������������ʡ����ʵķ��롢��ѧ����ʽ����д���Ѷ��еȣ�

 ��У����ϵ�д�
��У����ϵ�д�| A�� | AgCl�ǻ�ԭ���� | |
| B�� | ������Ӧʽ��Ag+Cl--e-=AgCl | |
| C�� | ÿ����1molNa2Mn5O10ת��2 mol���� | |
| D�� | Na+������ˮ����صĸ����ƶ� |
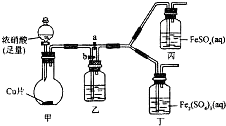 ijѧ����ͨ��ʵ�鷽����֤Fe2+�����ʣ�
ijѧ����ͨ��ʵ�鷽����֤Fe2+�����ʣ���1����ͬѧ��ʵ��ǰ������Fe2+�Ļ�ԭ�ԣ���д���±���
| ʵ����� | Ԥ������ | ��Ӧ�����ӷ���ʽ |
| ��ʢ������FeSO4��Һ���Թ��е�������Ũ���ᣬ�� | �Թ��в�������ɫ���壬��Һ��ɫ��� | Fe2++NO3-+2H+=Fe3++NO2+H2O |
��2����ԭ����FeSO4��Һ�з�Ӧ����Һ�о�����KSCN��Һ��ǰ�߲����ɫ�����߱�죮������Ľ�����Fe2+����������ΪFe3+��
��3����ͬѧͨ���������ϣ���Ϊ��Һ�ĺ���ɫ������NO2��NO����Һ��Fe 2+��Fe 3+������Ӧ���õ��ģ�Ϊ����������ͼװ�ã��������Ѽ��飬β������װ���ԣ�����̽����
����a���ر�b����ʹ��װ���з�Ӧ��ʼ�۲쵽������Һ��Ϊ����ɫ����������Һ�����Ա仯��
����b���ر�a��һ��ʱ�����ֹͣ���з�Ӧ��
��Ϊ�����ʵ����ж��գ�������������ʹ���з�Ӧ�������۲쵽�������벽�������ͬ��
��ͭ������Ũ���ᷴӦ�Ļ�ѧ����ʽ��Cu+4HNO3��Ũ��=Cu��NO3��2+NO2��+2H2O��
��װ���ҵ�������ʹNO2ת��ΪNO��
�۲�����Ŀ�����ų����Ҳ�װ���в�����NO2��
�ܸ�ʵ��ɵó��Ľ�������Һ������ɫ����Fe2+��NO��NO2���õõ���
��4����ͬѧ���½��У�����ʵ�飬�۲쵽��Ԥ��������ʵ���������ʢ��Ũ������Թ��е����������Ƶ�FeSO4��Һ����
| A�� | ���Բ��ܽ�������Dz����ڵ� | |
| B�� | ������ˮ���������ܽ��Ϊ�� | |
| C�� | ij���ӱ�������ȫ�������������Һ�е�Ũ��Ϊ�� | |
| D�� | ���ʵ��ܽ���Ϊ���ܣ�������ʲ�����ˮ |
| A�� | �����õ�����Һ����ӵ������Ƿ�Ԫ�� | |
| B�� | ˮ������觵���Ҫ�ɷ��Ƕ������� | |
| C�� | ����ɫ��Ⱦ����ָ�ɲ��ɽ����������ɵĻ�����Ⱦ | |
| D�� | ��͵������������γ��������Ҫ���� |
| A�� | �������һ�����Ӳ� | B�� | ���ǵĻ�ѧ�������� | ||
| C�� | ��ԭ�ӣ������Ӿ�ΪͬһԪ�� | D�� | ���ǵ�������������ͬ |
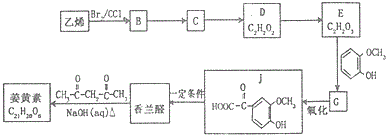

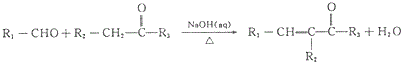 ��R1��R2��R3Ϊ��������ԭ�ӣ�
��R1��R2��R3Ϊ��������ԭ�ӣ�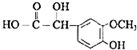 ��
��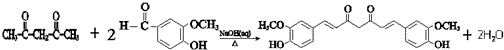 ��
��