��Ŀ����
��10�֣�ǰ20��Ԫ��A��B��C��D��AԪ��������������������������ԭ����������ȣ�B��ԭ�Ӱ뾶����������������С�ģ�B������������Ӧˮ����Ļ�ѧʽΪHBO3��CԪ��ԭ�ӵ������������ȴ������2����C����������D�������Ӿ�����ͬ�ĵ����Ų�����Ԫ�ؿ��γɻ�����D2C��
(1) BԪ�ص�ԭ�ӽṹʾ��ͼ ��
(2)C�����ڱ��е�λ�� ��
(3)������D2C�ĵ���ʽ__��
(4) B���⻯���ˮ��Һ����B������������Ӧˮ�����ϡ��Һ����Ӧ�����ӷ���ʽΪ�� ��
(5)������Ϊ��A������һ��������Դ������Ϊ����Ϊ��Դ���ŵ��ǣ�
(1) BԪ�ص�ԭ�ӽṹʾ��ͼ ��
(2)C�����ڱ��е�λ�� ��
(3)������D2C�ĵ���ʽ__��
(4) B���⻯���ˮ��Һ����B������������Ӧˮ�����ϡ��Һ����Ӧ�����ӷ���ʽΪ�� ��
(5)������Ϊ��A������һ��������Դ������Ϊ����Ϊ��Դ���ŵ��ǣ�
(1) ��N��ԭ�ӽṹʾ��ͼ����2�֣���
(2) �������ڵڢ�A�� ��2�֣���(3)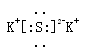 ��2�֣���
��2�֣���
(4) NH3��H2O + H+ = NH4+ + H2O ��2�֣���
��5����ֵ�ߣ���Դ�㣻����Ⱦ����2�֣���һ����1�֣�
(2) �������ڵڢ�A�� ��2�֣���(3)
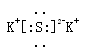 ��2�֣���
��2�֣��� (4) NH3��H2O + H+ = NH4+ + H2O ��2�֣���
��5����ֵ�ߣ���Դ�㣻����Ⱦ����2�֣���һ����1�֣�
ǰ20��Ԫ����ֻ����Ԫ�ص�������������������ԭ����������ȣ�AΪ�⣻BԪ����HBO3���ϼ���+5�������ǵ�������Ԫ�أ�������������뾶��С�ģ�BΪ��Ԫ�أ�Cֻ������Ԫ�أ�����D2C֪D�Ļ��ϼ���+1�ۣ�C����������D�������Ӿ�����ͬ�ĵ����Ų�������D�Ǽ�Ԫ�ء�������������������⡣

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ

 ����Ӧ�����γ�
����Ӧ�����γ� ������103.9k!����÷�Ӧ��
������103.9k!����÷�Ӧ��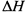 =_______��
=_______��