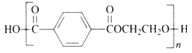
��Ŀ����
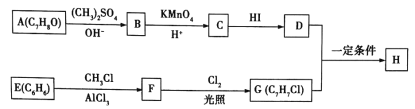
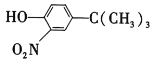
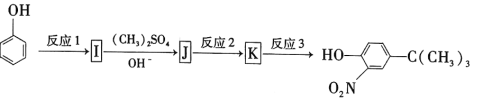
����Ŀ����֪A��һ����̬��,��Է���������28,ͨ��������ʵ�����,�����������������һ������ʯ�ͻ�����չˮƽ��D�dz�����ij�ֵ�ζƷ�ijɷ�֮һ, �����ʼ��ת����ϵ����ͼ(��Ӧ������ʡ��)���ش���������:
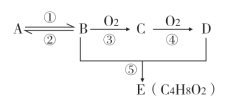
(1)B���ʵ�����________________.
(2)D�����к��еĹ���������________________________.
(3)C���ʵĽṹ��ʽ________________________
(4)��Ӧ�ݷ���������________________________
(5)��Ӧ�ܵķ�Ӧ����________________________
(6)��Ӧ�ٵĻ�ѧ����ʽ:________________________________
���𰸡��Ҵ� �Ȼ� CH3CHO Ũ���ᡢ���� ������Ӧ CH2=CH2+H2O![]() CH3CH2OH
CH3CH2OH
��������
A��һ����̬������Է���������28��ͨ��������ʵ������������������������һ������ʯ�ͻ�����չˮƽ����AΪCH2=CH2��D�dz�����ij�ֵ�ζƷ�ijɷ�֮һ���ٽ��ת����ϵ��֪DӦΪCH3COOH����BΪ��ϩ��ˮ�ļӳɲ���CH3CH2OH��CΪCH3CHO��EΪCH3CH2OOCCH3��
(1)BΪCH3CH2OH��������Ϊ�Ҵ���
(2)DΪCH3COOH���������Ϊ�Ȼ���
(3)���ݷ�����֪CΪCH3CHO��
(4)������Ҵ���Ũ���ᡢ���ȵ���������������������
(5)��Ӧ��Ϊ��ȩ�Ĵ�����������������Ӧ��
(6)��Ӧ��Ϊ��ϩ��ˮ�ļӳɷ�Ӧ������ʽΪ��CH2=CH2+H2O![]() CH3CH2OH��
CH3CH2OH��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�