��Ŀ����
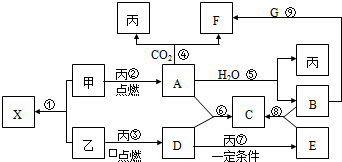
�ס��ҡ���Ϊ�������ʣ��ҡ�����Ԫ�������ڱ���λ��ͬ���塣X��A��B��C��D��E��F��G��Ϊ�����Ļ��������A��X��Ħ��������ͬ��A��F��C��G����ɫ��Ӧ��Ϊ��ɫ����һ�������£��������ת����ϵ����ͼ��������������⣺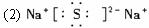
(1)�û�ѧʽ��ʾ����Ϊ__________________��EΪ__________________��
(2)X�ĵ���ʽΪ__________________��
(3)A��H2O��Ӧ�Ļ�ѧ����ʽΪ__________________��
(4)B��G��Һ��Ӧ����F�����ӷ�Ӧ����ʽΪ__________________��
(5)�١��ᷴӦ�У�������������ԭ��Ӧ����(�����) __________________��
(1)O2 SO3
![]()
(3)2Na2O2+2H2O![]() 4NaOH+O2��
4NaOH+O2��
(4) ![]() +
+![]()
![]() +H2O
+H2O
(5)���
������������֪��A��F��C��G��Ϊ�ƵĻ�����ɷ�Ӧ������֪����ΪO2����AΪ�������A+CO2��O2+F����֪AΪNa2O2����FΪNa2CO3����ΪA��X��Ħ��������ȣ���֪XΪNa2S������ΪS���ʣ�DΪSO2��

��ϰ��ϵ�д�
 �¿α�ͬ��ѵ��ϵ�д�
�¿α�ͬ��ѵ��ϵ�д� һ����ʦ����Ӧ����������һ��ȫϵ�д�
һ����ʦ����Ӧ����������һ��ȫϵ�д�
�����Ŀ