��Ŀ����
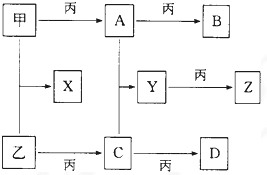
��֪�ס��ҡ���Ϊ�������ʣ�A��B��C��D��X��Y��ZΪ�����Ļ����Y��Ħ��������ֵ��Z��С16���ҡ�����Ħ��������ͬ��B��Ħ��������D��С2��B��X��Ħ��������ͬ��������ת����ϵ���£�
��1��д�����Ļ�ѧʽ ��B�ĵ���ʽ ��
��2��д��Y��Z�Ļ�ѧ����ʽ��
��3��ʵ��������Y��Һʱ�ױ��ʣ���д������Y��Һ�Ƿ���������Լ��� ��

��1��д�����Ļ�ѧʽ
��2��д��Y��Z�Ļ�ѧ����ʽ��
��3��ʵ��������Y��Һʱ�ױ��ʣ���д������Y��Һ�Ƿ���������Լ���
��������Y��Ħ��������ֵ��Z��С16�����ݿ�֪����ΪO2���ҡ�����Ħ��������ͬ������ΪS������ת����ϵ��֪��CΪSO2��DΪSO3��������������A��A����������B��B��Ħ��������D��С2��B��X��Ħ��������ͬ����B��X��Ħ������Ϊ78g/mol�����ǿ��ܼ�ΪNa��AΪNa2O��BΪNa2O2����XΪNa2S����Ħ������Ϊ78g/mol��A��C��Ӧ����YΪNa2SO3����ZΪNa2SO4������ת����ϵ���ݴ˽��
����⣺��Y��Ħ��������ֵ��Z��С16�����ݿ�֪����ΪO2���ҡ�����Ħ��������ͬ������ΪS������ת����ϵ��֪��CΪSO2��DΪSO3��������������A��A����������B��B��Ħ��������D��С2��B��X��Ħ��������ͬ����B��X��Ħ������Ϊ78g/mol�����ǿ��ܼ�ΪNa��AΪNa2O��BΪNa2O2����XΪNa2S����Ħ������Ϊ78g/mol��A��C��Ӧ����YΪNa2SO3����ZΪNa2SO4������ת����ϵ��
��1��������������֪����ΪO2��BΪNa2O2�������ʽΪ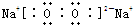 ��
��
�ʴ�Ϊ��O2��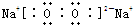 ��
��
��2��Y��Z���������Ʊ������������������ƣ���ѧ����ʽΪ��2Na2SO3+O2�T2Na2SO4��
�ʴ�Ϊ��2Na2SO3+O2�T2Na2SO4��
��3��ʵ��������Na2SO3��Һʱ�ױ��ʣ�����Na2SO4��ͨ�������Ƿ���������ж��Ƿ���ʣ������Լ��ǣ�ϡ������Ȼ�����Һ��
�ʴ�Ϊ��ϡ������Ȼ�����Һ��
��1��������������֪����ΪO2��BΪNa2O2�������ʽΪ
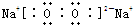 ��
���ʴ�Ϊ��O2��
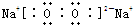 ��
����2��Y��Z���������Ʊ������������������ƣ���ѧ����ʽΪ��2Na2SO3+O2�T2Na2SO4��
�ʴ�Ϊ��2Na2SO3+O2�T2Na2SO4��
��3��ʵ��������Na2SO3��Һʱ�ױ��ʣ�����Na2SO4��ͨ�������Ƿ���������ж��Ƿ���ʣ������Լ��ǣ�ϡ������Ȼ�����Һ��
�ʴ�Ϊ��ϡ������Ȼ�����Һ��
���������⿼�������ƶϣ���Ҫ������ø����ʵ�Ħ��������ϵ���ƶϱ�Ϊ�����ǹؼ���Ӧ����ע��Ԫ�ػ�����֪ʶ��

��ϰ��ϵ�д�
 ��ĩ��ϰ���ϵ�д�
��ĩ��ϰ���ϵ�д� ����ѧ�䵥Ԫ������ĩר����100��ϵ�д�
����ѧ�䵥Ԫ������ĩר����100��ϵ�д� �Ƹ�360�ȶ����ܾ�ϵ�д�
�Ƹ�360�ȶ����ܾ�ϵ�д� ���⿼����Ԫ���Ծ�ϵ�д�
���⿼����Ԫ���Ծ�ϵ�д�
�����Ŀ



