��Ŀ����
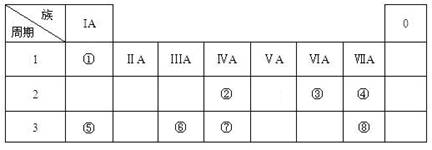
��֪���ס��ҡ���Ϊ�������ʣ��׳�����Ϊ���壬��Ϊһ����ɫ���壬��Ϊ��ɫ���壮A��B��C��D��Ϊ�����DΪһ���ɫ����������֮������ͼת����ϵ����ش��������⣮

��1��д���������ʵĻ�ѧʽ����
��2��д���۷�Ӧ�Ļ�ѧ����ʽ��
��3��д���ڷ�Ӧ�����ӷ���ʽ��

��1��д���������ʵĻ�ѧʽ����
Cl2
Cl2
��BFeCl2
FeCl2
����2��д���۷�Ӧ�Ļ�ѧ����ʽ��
4Fe��OH��2+2H2O+O2=4Fe��OH��3
4Fe��OH��2+2H2O+O2=4Fe��OH��3
����3��д���ڷ�Ӧ�����ӷ���ʽ��
Fe+2Fe3+=3Fe2+
Fe+2Fe3+=3Fe2+
��������������Ϊһ����ɫ��������Ϊ������DΪһ���ɫ����˵��DΪ�������������ת��ͼD��A���������Ʒ�Ӧ���ɣ�����Aһ���Ǻ����Ļ�����ƶϼ�Ϊ��������AΪ�Ȼ�����A�ͼ�Ӧ����B�����Ȼ���������Ӧ������BΪ�Ȼ�������B���������Ʒ�Ӧ����CΪ����������������C�ͱ����巴Ӧ����D�������������Ա�Ϊ�������ۺ�ת����ϵ�����ƶϸ����ʵ���ɺͷ����Ļ�ѧ��Ӧ��
����⣺����Ľ���ؼ�����Ϊһ����ɫ����ֱ���ж�Ϊ������DΪһ���ɫ�����ж�Ϊ������������ϼס��ҡ���Ϊ�������ʣ��׳�����Ϊ���壬��Ϊ��ɫ���壮A��B��C��D��Ϊ�����ͨ��ת����ϵͼ��һ�����жϸ����ʵ���ɺͷ�Ӧ����D������������֪��A+NaOH��Fe��OH��3���ƶ�A�Ǻ������Ȼ��ȷ��AΪFeCl3����ΪFe��ת��ͼ�� A+�ס�B���Ƿ�����Fe+2Fe3+=3Fe2+��B+NaOH��C����������Fe2++2OH-=Fe��OH��2����C+����D�������ķ�Ӧ�ǣ�4Fe��OH��2+2H2O+O2=4Fe��OH��3��
����������ת����ϵ�еĸ����ʷֱ�Ϊ��
�ף�Fe���ң�Cl2������O2��A��FeCl3��B��FeCl2��C��Fe��OH��2��D��Fe��OH��3��
���������������жϻش��������⣺
��1���ҵĻ�ѧʽΪCl2��B�Ļ�ѧʽFeCl2��
�ʴ�Ϊ��Cl2 FeCl2
��2���ڢ۷�Ӧ�Ļ�ѧ����ʽ�DZ���������Ϊ���������ķ�Ӧ������������ѧ����ʽΪ4Fe��OH��2+2H2O+O2=4Fe��OH��3
�ʴ�Ϊ��4Fe��OH��2+2H2O+O2=4Fe��OH��3
��3���ڢڷ�Ӧ�����ӷ���ʽ���Ȼ�������������������ԭ��Ӧ����Һ�з�Ӧ�����ӷ���ʽΪ��Fe+2Fe3+=3Fe2+��
�ʴ�Ϊ��Fe+2Fe3+=3Fe2+
����������ת����ϵ�еĸ����ʷֱ�Ϊ��
�ף�Fe���ң�Cl2������O2��A��FeCl3��B��FeCl2��C��Fe��OH��2��D��Fe��OH��3��
���������������жϻش��������⣺
��1���ҵĻ�ѧʽΪCl2��B�Ļ�ѧʽFeCl2��
�ʴ�Ϊ��Cl2 FeCl2
��2���ڢ۷�Ӧ�Ļ�ѧ����ʽ�DZ���������Ϊ���������ķ�Ӧ������������ѧ����ʽΪ4Fe��OH��2+2H2O+O2=4Fe��OH��3
�ʴ�Ϊ��4Fe��OH��2+2H2O+O2=4Fe��OH��3
��3���ڢڷ�Ӧ�����ӷ���ʽ���Ȼ�������������������ԭ��Ӧ����Һ�з�Ӧ�����ӷ���ʽΪ��Fe+2Fe3+=3Fe2+��
�ʴ�Ϊ��Fe+2Fe3+=3Fe2+
���������⿼�������������������Ļ������ ��ѧ���ʵ�Ӧ�ú������жϣ��ؼ������ʵ�������ɫ��������Ӧ���ҵ��ƶ����ʵ������ǽ�������ͻ�ƿڣ�

��ϰ��ϵ�д�
 �ִʾ�ƪ��ͬ�����Ĵ��ϵ�д�
�ִʾ�ƪ��ͬ�����Ĵ��ϵ�д�
�����Ŀ