��Ŀ����
��18.4 mol��L��1��Ũ��������100mL1.0mol��L��1ϡ���ᡣ
��1������ȡŨ��������Ϊ mL������һλС������
��2����ʵ�������У�
��A��100mL��Ͳ ��B��������ƽ ��C�������� ��D��50mL����ƿ ��E��10mL��Ͳ ��F����ͷ�ι� ��G��50mL�ձ� ��H��100mL����ƿ
ʵ��ʱѡ�õ�������C��F��G�Ӧѡ�ã�����ţ�
��3�����ƹ����У����������ʹ���ƽ��ƫ�ߵ��ǣ�����ţ�
��A������ʱ���ӿ̶��߹۲�Һ�棻
��B������ƿʹ��ʱδ���
��C�����ݺ���ҡ�ȡ����ã�����Һ����ڿ̶��ߣ��ټ�����ˮ�����̶���
��4��������ƿ��ʹ���У����в�������ȷ���ǣ�����ţ�
��A��ʹ������ƿǰ������Ƿ�©ˮ
��B������ƿ������ˮϴ�����ô����Ƶ���Һϴ��
��C�����������ƹ��������ƽ���̵���ֽ�ϣ�ȷ�����������ձ����ܽ������ע������ƿ��
��D����ȷ��ȡ��18.4mol��L��1�����ᣬע����ʢ��30mLˮ��100mL������ƿ�У���ˮ���̶���
��E�����ݺ�����ƿ������ʳָ��סƿ��������һֻ�ֵ���ָ��סƿ�ף�������ƿ��תҡ��
��1������ȡŨ��������Ϊ mL������һλС������
��2����ʵ�������У�
��A��100mL��Ͳ ��B��������ƽ ��C�������� ��D��50mL����ƿ ��E��10mL��Ͳ ��F����ͷ�ι� ��G��50mL�ձ� ��H��100mL����ƿ
ʵ��ʱѡ�õ�������C��F��G�Ӧѡ�ã�����ţ�
��3�����ƹ����У����������ʹ���ƽ��ƫ�ߵ��ǣ�����ţ�
��A������ʱ���ӿ̶��߹۲�Һ�棻
��B������ƿʹ��ʱδ���
��C�����ݺ���ҡ�ȡ����ã�����Һ����ڿ̶��ߣ��ټ�����ˮ�����̶���
��4��������ƿ��ʹ���У����в�������ȷ���ǣ�����ţ�
��A��ʹ������ƿǰ������Ƿ�©ˮ
��B������ƿ������ˮϴ�����ô����Ƶ���Һϴ��
��C�����������ƹ��������ƽ���̵���ֽ�ϣ�ȷ�����������ձ����ܽ������ע������ƿ��
��D����ȷ��ȡ��18.4mol��L��1�����ᣬע����ʢ��30mLˮ��100mL������ƿ�У���ˮ���̶���
��E�����ݺ�����ƿ������ʳָ��סƿ��������һֻ�ֵ���ָ��סƿ�ף�������ƿ��תҡ��
��1��5.4 ��2��EH ��3��A ��4��CD
��

��ϰ��ϵ�д�
 ���ɿ��õ�Ԫ������ĩר����100��ϵ�д�
���ɿ��õ�Ԫ������ĩר����100��ϵ�д�
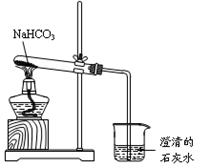
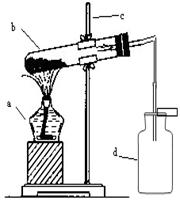
�����Ŀ
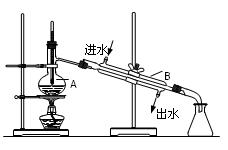
 00mL0.2000 mol��L-1Na2CO3��Һ����ƿ��,��2��3�μ�����ָʾ������δ֪Ũ�ȵ��������ζ�0.2000 mol��L-1Na2CO3��Һ������ �жϵζ��յ�ﵽ��
00mL0.2000 mol��L-1Na2CO3��Һ����ƿ��,��2��3�μ�����ָʾ������δ֪Ũ�ȵ��������ζ�0.2000 mol��L-1Na2CO3��Һ������ �жϵζ��յ�ﵽ�� �����ߵ�ƽ��ֵΪ3.41�棬��ʵ�����к��ȡ�H= (��Ϻ���Һ�ı�����C = 4.18J����-1��g-1)��ʵ�����к��ȱ����� (ƫ�ߣ���ȣ�ƫ��)
�����ߵ�ƽ��ֵΪ3.41�棬��ʵ�����к��ȡ�H= (��Ϻ���Һ�ı�����C = 4.18J����-1��g-1)��ʵ�����к��ȱ����� (ƫ�ߣ���ȣ�ƫ��)