��Ŀ����
����Ŀ��������������ȷ�ĸ����У� ��
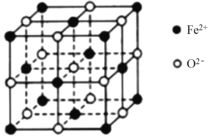
����ԭ�ӵĵ����Ų�ͼ��![]() �����Υ��������ԭ��
�����Υ��������ԭ��
�ڴ����������״̬ԭ�ӽл�̬ԭ�ӣ�1s22s22px1��1s22s22py1�������γɵ��Ƿ������
������Ԫ���У����ĵ�һ���������
�ܾ�����ͬ��������Ų������ӣ���ѧ������ͬ
��NCl3��N-Cl���ļ�����CCl4��C-Cl���ļ�����
����Ԫ�صĵ縺�Ծ�С��1.8
������Ԫ�صĵ縺��Խ����Ԫ��ԭ�ӵĵ�һ������һ��Խ��
�����κ�����£����ǦҼ��Ȧм�ǿ�ȴ�
A.0��B.1��C.2��D.3��
���𰸡�B
��������
������������ԭ���ֳ�����ԭ��,��ָȷ����һ��ԭ�ӹ�������������������ӣ������������ӵ�������������෴��ͬʱҪ��ѭ���ع������ӷֲ���ԭ�ӹ��ʱ��������������ͬ�ķ�ʽ�ֱ�ռ�ݲ�ͬ�Ĺ������Ϊ�����Ų���ʽԭ�ӵ���������͡�����ԭ�ӵĵ����Ų�ͼ��![]() �����Υ���˺��ع���������
�����Υ���˺��ع���������
�������������״̬ԭ�ӽл�̬ԭ�ӣ����ڸ��ܼ���ԭ�ӻ��������ϵ��ܼ�ԾǨʱ�������䣬����������������ȥ�γɵĹ��з�����ף�1s22s22px1��1s22s22py1�����У�����p�ܼ���������ͬ�Ĺ����������С��ͬ����px = py = pz�������γɵķ�����ף���������
��ϡ�������ԭ�ӽṹ���ȶ��ṹ��ͬ����ϡ������ĵ�һ���������ͬ�����϶��µ�һ�����ܽ��ͣ��ʺ�Ԫ�صĵ�һ���������������
����������Ų���ȫ��ͬ����������һ������ͬ��Ԫ�أ���ѧ���ʲ�һ����ͬ����Na+��F-��������Ų���ͬ�����Ƕ���ѧ���ʲ�ͬ����������
����Ԫ�غ�̼Ԫ��ͬ���ڣ�ͬ���ڴ�������ԭ�Ӱ뾶���μ�С����ԭ�Ӱ뾶С��̼ԭ�Ӱ뾶����NCl3��N-Cl���ļ�����CCl4��C-Cl���ļ����̣�������ȷ��
������Ԫ�صĵ縺��һ��С��1.8���ǽ���Ԫ�صĵ縺��һ�����1.8����λ�ڷǽ����������߽�����������(���ࡢ���)�ĵ縺������1.8���ң����н������зǽ�������������
������Ԫ��ԭ�ӵĵ�һ�����ܡ��縺�Ա仯���ƻ�����ͬ���������ܱ仯����������縺�ԣ�O��N������һ�����ܣ�O��N����������
���м����Ŀռ乹���Ǹ��ݵ��ӹ���ġ��粢�硱���ص���һ��һ�����ʦҼ��ͦм������ڵ�ʱ������Ӧʱ�������ȶϣ�����������ǿ�ȴ���N2�����д��ڵ�N��N���ܱ�3��N-N���ܴ�Ҳ��һ��N-N��һ��N=N���ܼ�����Ҫ��˵��N��N�е�����������ǿ����������
��ѡB��

 �������ͬ������ϵ�д�
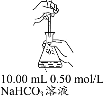
�������ͬ������ϵ�д�����Ŀ����10.00 mL 0.50 mol/L NaHCO3��Һ�еμӲ�ͬŨ�ȵ�CaCl2��Һ���۲쵽���Բ�������ʱ��ֹͣ�μӣ�ȡ�������û���Һ���ȣ���¼ʵ����������˵������ȷ����
ʵ�� | ��� | c(CaCl2)(mol��L-1) | �μ�CaCl2��Һʱ�� ʵ������ | ���Ȼ���Һʱ�� ʵ������ |
| �� | 0.05 | ��1.32 mLʱ�������Ի��ǣ��������ݲ��� | �н϶��������� |
�� | 0.005 | ��15.60 mLʱ�������Ի��ǣ��������ݲ��� | �������������� | |
�� | 0.0005 | ��20 mL����� |
A.���в������ǵ�ԭ����c(Ca2+)��c(CO32)>Ksp(CaCO3)
B.δ����ǰ�ٺ͢��з����˷�Ӧ��2HCO3-��Ca2+=CaCO3����H2CO3
C.������Һ����������Ҫ����ΪCaCO3���ȷֽ�����˸����CO2
D.������NaHCO3��Һ�м�������0.5 mol/LCaCl2��Һ������ͬʱ�������Ǻ�����