��Ŀ����
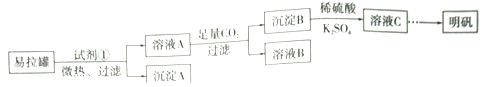
����Ŀ������[KAl(SO4)2��12H2O]��һ���Σ�����ֽ�ȷ���Ӧ�ù㷺��ij��ȤС�����10.0g��������(��90%��Al��������������Fe��Mg�����ʣ��Ʊ�������ʵ�鷽�����£�
��1���Լ���Ӧѡ��_____________(����ţ���
a.���� b.H2SO4��Һ c.�Ȼ�����Һ d.NaOH��Һ
��2���������ܽ��������Ҫ��Ӧ�Ļ�ѧ����ʽΪ____________________��
��3����ҺB�����ʵ���Ҫ�ɷ�Ϊ____________________ (�ѧʽ)��
��4������ҺC�еõ�������ʵ���������Ϊ����Ũ����__________(��������ƣ������ˡ�ϴ�ӡ��������ͼ��ʾ�����������е�һ��������_____________��
��5����С����ʵ�����֮�õ�118.5 g�������������Ļ�����Ϊ_____________������֪��������Ħ������Ϊ474g��mol-1)
���𰸡� d 2Al+2NaOH+2H2O=2NaAlO2+3H2�� NaHCO3 ��ȴ�ᾧ ����ʱ����ʹ������(������ʱӦʹ��Ӧ������) 75%
����������������Ҫ�ɷ�ΪAl������������Fe��Mg���ʣ���ѡ��ŨNaOH�ܽ⣬�õ�ƫ��������Һ����ҺA������ͨ�����˳�ȥFe��Mg������A�������ʣ���Һ��ͨ��CO2��Һ��������������������̼�����ƣ�ͨ�����˵õ�̼��������Һ����ҺB������������������B�������˺����ܽ���ϡ�����У��õ���������Һ������K2SO4��Һ������Ũ������ȴ�ᾧ�õ��������塣��
��1�����������ܽ���ǿ���ǿ������Һ��������þֻ���ܽ���ǿ������Һ�е����ʲ��죬��ѡ��NaOH��Һ�ܽ������ޣ��ɳ�ȥ���е�����þ�����ʣ���ѡd����2����������������Һ��Ӧ����ƫ�����ƺ�������������Ӧ�Ļ�ѧ����ʽΪ2Al+2NaOH+2H2O��2NaAlO2+3H2������3�����ݷ�����֪��ƫ��������Һ��ͨ��CO2��Һ��������������������̼�����ƣ�ͨ�����˵õ�̼��������Һ����ҺB������������������B������ҺBΪNaHCO3��Һ����4����������Һ������K2SO4��Һ������Ũ������ȴ�ᾧ�����ˡ�ϴ�ӡ�����õ��������壻��������ʹ��������Ӧ��������������2��������Ԫ���غ��֪�����Ͽɵõ�����������Ϊ��10g��90%��474/27=158g���������Ļ�����Ϊ118.5/158��100%=75%��

����Ŀ����1x105Pa��298 Kʱ����1 mol��̬AB���ӷ������̬Aԭ�Ӻ�Bԭ������Ҫ��������ΪA-B���ۼ��ļ���( kJ/mol)��������һЩ���ۼ��ļ��ܣ���֪����������3���ȼ۵ĵ���ۼ�����
���ۼ� | H-H | N��N | N-H |
���ܣ�kJ/mol�� | 436 | 945 | 391 |
��1�������ϱ��е������жϹ�ҵ�ϳɰ��ķ�Ӧ��N2(g)+3H2(g)![]() 2NH3(g)��____��������������������������Ӧ��
2NH3(g)��____��������������������������Ӧ��
��2����298 Kʱ��ȡ1mol������3mol��������һ�ܱ������У����ڴ�������������ǡ����ȫ��Ӧ�������Ϸų������յ�����ΪQ1��Q1����ֵ��____��
��3��ʵ�������У��ų������յ�����ΪQ2������ѡ����ȷ����____����ѡ����ţ���
A��Q1>Q2 B��Q1<Q2 C��Q1=Q2
��������ѡ���ԭ����________��