��Ŀ����
��1���й��Ŵ��Ĵ���֮һ--�ڻ�ҩ�����ı�ը��ӦΪ��
2KNO3+3C+S
A+N2��+3CO2��������ƽ��
�ٳ�S�⣬����Ԫ�صĵ縺�ԴӴ�С����Ϊ
�����������У�A�ľ�������Ϊ
����֪CN-��N2Ϊ�ȵ����壬����HCN�����ЦҼ���м���Ŀ֮��Ϊ
��2��ԭ������С��36��Ԫ��Q��T�������ڱ��мȴ���ͬһ������λ��ͬһ�壬��ԭ������T��Q��2��T�Ļ�̬ԭ����Χ���ӣ��۵��ӣ��Ų�Ϊ
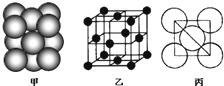
��3����ij�������ʾ�����ԭ�ӵĶѻ���ʽ��ͼ����ʾ���侧��������ͼ����ʾ��ԭ��֮���λ�ù�ϵ��ƽ��ͼ��ͼ����ʾ�����и�ԭ�ӵ���λ��Ϊ
��4����CrCl3��ˮ��Һ�У�һ�������´������Ϊ[CrCln��H2O��6-n]x+��n��x��Ϊ���������������ӣ�����ͨ�������ӽ�����֬��R-H�����ɷ������ӽ�����Ӧ��[CrCln��H2O��6-n]x++xR-H-��Rx[CrCln��H2O��6-n]x++xH+����������H+���к͵ζ����������x��n��ȷ�������ӵ���ɣ�����0.0015mol[CrCln��H2O��6-n]x+����Һ����R-H��ȫ�������к����ɵ�H+��Ũ��Ϊ0.1200mol?L-1NaOH��Һ25.00mL����֪�������ӵĻ�ѧʽΪ
2KNO3+3C+S
| ||
�ٳ�S�⣬����Ԫ�صĵ縺�ԴӴ�С����Ϊ
O��N��C��K
O��N��C��K
�������������У�A�ľ�������Ϊ
���Ӿ���
���Ӿ���
�������Թ��ۼ��ķ��ӵ�����ԭ�ӹ���ӻ�����Ϊsp�ӻ�
sp�ӻ�
������֪CN-��N2Ϊ�ȵ����壬����HCN�����ЦҼ���м���Ŀ֮��Ϊ
1��1
1��1
����2��ԭ������С��36��Ԫ��Q��T�������ڱ��мȴ���ͬһ������λ��ͬһ�壬��ԭ������T��Q��2��T�Ļ�̬ԭ����Χ���ӣ��۵��ӣ��Ų�Ϊ
3d84s2
3d84s2
��Q2+��δ�ɶԵ�������4
4
����3����ij�������ʾ�����ԭ�ӵĶѻ���ʽ��ͼ����ʾ���侧��������ͼ����ʾ��ԭ��֮���λ�ù�ϵ��ƽ��ͼ��ͼ����ʾ�����и�ԭ�ӵ���λ��Ϊ
12
12
���õ��ʾ�����ԭ�ӵĶѻ���ʽΪ���ֻ����ѻ���ʽ�е�ͭ��
ͭ��
��
��4����CrCl3��ˮ��Һ�У�һ�������´������Ϊ[CrCln��H2O��6-n]x+��n��x��Ϊ���������������ӣ�����ͨ�������ӽ�����֬��R-H�����ɷ������ӽ�����Ӧ��[CrCln��H2O��6-n]x++xR-H-��Rx[CrCln��H2O��6-n]x++xH+����������H+���к͵ζ����������x��n��ȷ�������ӵ���ɣ�����0.0015mol[CrCln��H2O��6-n]x+����Һ����R-H��ȫ�������к����ɵ�H+��Ũ��Ϊ0.1200mol?L-1NaOH��Һ25.00mL����֪�������ӵĻ�ѧʽΪ
[CrCl��H2O��5]2+
[CrCl��H2O��5]2+
���������ӵ���λ��Ϊ6
6
����������1����ͬ����������ҵ縺����������Խǿ�縺��ԽС���ݴ˽��
����ԭ���غ��֪������AΪK2S���ɼ������������ӹ��ɣ�
�����Թ��ۼ��ķ���ΪCO2��������Cԭ���γ�2��C=O���������¶Ե��ӣ��ӻ������ĿΪ2��
��CN-��N2�ṹ���ƣ�Cԭ����Nԭ��֮���γ���������HCN���ӽṹʽΪH-C��N�������к���1���Ҽ���2���м����������ڦҼ���
��2��ԭ������С��36��Ԫ��Q��T�������ڱ��мȴ���ͬһ������λ��ͬһ�壬��Q��T���ڵڢ��壬��ԭ������T��Q��2����QΪFeԪ�أ�TΪNiԪ�أ��پ�����������Ų����ɽ��
��3����ԭ�ӵ���λ��=3��8��
=12���þ�������Ϊ����������
��4�����к����ɵ�H+��Ҫ��NaOH��Һ���ɵó�H+���ʵ��������������x���ٽ��Cr�Ļ��ϼ�+3�ۣ���n��
����ԭ���غ��֪������AΪK2S���ɼ������������ӹ��ɣ�
�����Թ��ۼ��ķ���ΪCO2��������Cԭ���γ�2��C=O���������¶Ե��ӣ��ӻ������ĿΪ2��
��CN-��N2�ṹ���ƣ�Cԭ����Nԭ��֮���γ���������HCN���ӽṹʽΪH-C��N�������к���1���Ҽ���2���м����������ڦҼ���
��2��ԭ������С��36��Ԫ��Q��T�������ڱ��мȴ���ͬһ������λ��ͬһ�壬��Q��T���ڵڢ��壬��ԭ������T��Q��2����QΪFeԪ�أ�TΪNiԪ�أ��پ�����������Ų����ɽ��
��3����ԭ�ӵ���λ��=3��8��
| 1 |
| 2 |
��4�����к����ɵ�H+��Ҫ��NaOH��Һ���ɵó�H+���ʵ��������������x���ٽ��Cr�Ļ��ϼ�+3�ۣ���n��
����⣺��1����ͬ����������ҵ縺����������Խǿ�縺��ԽС���ʵ縺��O��N��C��K��
�ʴ�Ϊ��O��N��C��K��
����ԭ���غ��֪������AΪK2S���ɼ������������ӹ��ɣ��������Ӿ��壻
�����Թ��ۼ��ķ���ΪCO2��������Cԭ���γ�2��C=O���������¶Ե��ӣ��ӻ������ĿΪ2��Ϊsp�ӻ���ʽ��
�ʴ�Ϊ�����Ӿ��壻sp��
��CN-��N2�ṹ���ƣ�Cԭ����Nԭ��֮���γ���������HCN���ӽṹʽΪH-C��N�������к���1���Ҽ���2���м����������ڦҼ�����HCN�����ЦҼ���м���Ŀ֮��Ϊ1��1��
�ʴ�Ϊ��1��1��
��2��ԭ������С��36��Ԫ��Q��T�������ڱ��мȴ���ͬһ������λ��ͬһ�壬��Q��T���ڵڢ��壬��ԭ������T��Q��2����QΪFeԪ�أ�TΪNiԪ�أ�NiԪ����28��Ԫ�أ�Niԭ�Ӽ۵����Ų�ʽΪ3d84s2��Fe2+�ĺ�������Ų�ʽΪ1s24s22p63s23d6��3d�ܼ���4�������ӣ�
�ʴ�Ϊ��3d84s2��4��
��3����ԭ�ӵ���λ��=3��8��
=12���þ�������Ϊ����������Ϊͭ�ͣ�
�ʴ�Ϊ��12��ͭ�ͣ������������ѻ�����
��4���к����ɵ�H+��Ũ��Ϊ0.1200mol/L����������Һ25.00mL������Եó�H+�����ʵ���Ϊ0.12nol/L��25.00��10-3L=0.0030mol������x=
=2��
Cr�Ļ��ϼ�Ϊ+3�ۣ����[CrCln��H20��6-n]x+����3-n=2�����Ե�֪n=1�����������ӵĻ�ѧʽΪ[CrCl��H2O��5]2+���������ӵ���λ����1+5=6��
�ʴ�Ϊ��[CrCl��H2O��5]2+��6��
�ʴ�Ϊ��O��N��C��K��
����ԭ���غ��֪������AΪK2S���ɼ������������ӹ��ɣ��������Ӿ��壻
�����Թ��ۼ��ķ���ΪCO2��������Cԭ���γ�2��C=O���������¶Ե��ӣ��ӻ������ĿΪ2��Ϊsp�ӻ���ʽ��
�ʴ�Ϊ�����Ӿ��壻sp��
��CN-��N2�ṹ���ƣ�Cԭ����Nԭ��֮���γ���������HCN���ӽṹʽΪH-C��N�������к���1���Ҽ���2���м����������ڦҼ�����HCN�����ЦҼ���м���Ŀ֮��Ϊ1��1��
�ʴ�Ϊ��1��1��
��2��ԭ������С��36��Ԫ��Q��T�������ڱ��мȴ���ͬһ������λ��ͬһ�壬��Q��T���ڵڢ��壬��ԭ������T��Q��2����QΪFeԪ�أ�TΪNiԪ�أ�NiԪ����28��Ԫ�أ�Niԭ�Ӽ۵����Ų�ʽΪ3d84s2��Fe2+�ĺ�������Ų�ʽΪ1s24s22p63s23d6��3d�ܼ���4�������ӣ�
�ʴ�Ϊ��3d84s2��4��
��3����ԭ�ӵ���λ��=3��8��
| 1 |
| 2 |
�ʴ�Ϊ��12��ͭ�ͣ������������ѻ�����
��4���к����ɵ�H+��Ũ��Ϊ0.1200mol/L����������Һ25.00mL������Եó�H+�����ʵ���Ϊ0.12nol/L��25.00��10-3L=0.0030mol������x=
| 0.0030 |
| 0.0015 |
Cr�Ļ��ϼ�Ϊ+3�ۣ����[CrCln��H20��6-n]x+����3-n=2�����Ե�֪n=1�����������ӵĻ�ѧʽΪ[CrCl��H2O��5]2+���������ӵ���λ����1+5=6��
�ʴ�Ϊ��[CrCl��H2O��5]2+��6��
���������⿼���˾������йؼ��㡢��λ����ȷ�����縺�Դ�С���жϵ�֪ʶ�㣬�縺�Դ�С���жϡ��ӻ���ʽ���ж϶���ѧϰ�ص㣬Ϊ������㣬�Ѷ��еȣ�

��ϰ��ϵ�д�
 Сѧѧϰ�ð���ϵ�д�
Сѧѧϰ�ð���ϵ�д� Сѧͬ�����������ܾ�ϵ�д�
Сѧͬ�����������ܾ�ϵ�д�
�����Ŀ
��3����CrCl3��ˮ��Һ�У�һ�������´������Ϊ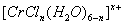 ��n��x��Ϊ���������������ӣ�����ͨ�������ӽ�����֬��R-H�����ɷ������ӽ�����Ӧ��
��n��x��Ϊ���������������ӣ�����ͨ�������ӽ�����֬��R-H�����ɷ������ӽ�����Ӧ��
[CrCl3(H2O)6-n]x+ +xR-H Rx[CrCl3(H2O)6-n]x+ +xH+
Rx[CrCl3(H2O)6-n]x+ +xH+
���������ľ��к͵ζ����������x��n,ȷ�������ӵ���ɡ�
����0��0015 mol ����Һ����R-H��ȫ�������к����ɵ���Ũ��Ϊ0��1200 mol��L-1 NaOH��Һ25��00 ml,�������ӵĻ�ѧʽΪ__________________ ��
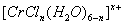 ��n��x��Ϊ���������������ӣ�����ͨ�������ӽ�����֬��R-H�����ɷ������ӽ�����Ӧ��
��n��x��Ϊ���������������ӣ�����ͨ�������ӽ�����֬��R-H�����ɷ������ӽ�����Ӧ�� [CrCl3(H2O)6-n]x+ +xR-H
 Rx[CrCl3(H2O)6-n]x+ +xH+
Rx[CrCl3(H2O)6-n]x+ +xH+���������ľ��к͵ζ����������x��n,ȷ�������ӵ���ɡ�
����0��0015 mol ����Һ����R-H��ȫ�������к����ɵ���Ũ��Ϊ0��1200 mol��L-1 NaOH��Һ25��00 ml,�������ӵĻ�ѧʽΪ__________________ ��
 ��1���й��Ŵ��Ĵ���֮һ--�ڻ�ҩ�����ı�ը��ӦΪ��
��1���й��Ŵ��Ĵ���֮һ--�ڻ�ҩ�����ı�ը��ӦΪ��