��Ŀ����
����Ŀ����1������ʽΪC5Hl2O����������Ʒ�Ӧ�ų�H2���л���������________�֣�
��2������˵���������_____________��
A.�л������ȼ��
B.�������Ա�̼�������ʱ��Ӳ�����̼������Һ��Ӧ
C.��ȡ��������ʱ���Լ��Ļ��˳�����ȼ�Ũ���ᣬ�ټ��Ҵ���Ȼ�������
D.ʯ�ͷ���õ��������Ǵ�����
��3������ʽΪC5H9C1O2��ͬ���칹�����࣬��������NaHCO3������Ӧ����CO2��ͬ���칹�干��________�֣�
��4������ʽΪC3H6O2����״�л���˴Ź��������Ϸ����Ŀ��ǿ�ȱȷֱ�Ϊ:��3��3����3��2��1;��3��1��1��1;��2��2��1��1�������ǿ��ܵĽṹ��ʽ����Ϊ��
��_______________����______________��
��_______________����__________________��
��5���������ᣨ![]() ���ж��������ͬ���칹�壬��������FeCl3��Һ������ɫ��Ӧ���ұ�������2��һ����ȡ�����ͬ���칹����_______________��д������2�ֵĽṹ��ʽ��
���ж��������ͬ���칹�壬��������FeCl3��Һ������ɫ��Ӧ���ұ�������2��һ����ȡ�����ͬ���칹����_______________��д������2�ֵĽṹ��ʽ��
��6����ϵͳ����������ͼ�������������___________
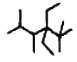
���𰸡� 8 ABCD 12 CH3COOCH3 CH3CH2COOH �� HCOOCH2CH3 �� CH3�DO�DCH2CHO CH3CH(OH)CHO HOCH2CH2CHO ![]() 2,2,4,5���ļ���3,3�����һ�����
2,2,4,5���ļ���3,3�����һ�����
����������1������ʽΪC5Hl2O����������Ʒ�Ӧ�ų�H2���л����������ڱ���һԪ����������C5H11��OH�������8�֣�����Ӧ��һԪ����8�֣���2��A.�л��ﲻһ������ȼ�գ��������Ȼ�̼���������A����B.�������Ա�̼��������ǿ��̼�����ƣ��ʱ�������̼������Һ��Ӧ���ɱ����ƺ�̼�����ƣ�B����C.Ũ��������ˮ�ų��������ȣ����ܶȱ�ˮ��Ϊ��ֹ��Һ�ɽ���˳��Ϊ�Ҵ���Ũ���ᡢ���ᣬ�ӱ��������ȼ���Ũ�����ټ����Ҵ������ᣬC����D.ʯ�ͷ���õ���������Ȼ�ǻ���D����ѡABCD����3������ʽΪC5H9ClO2���л�������NaHCO3������Ӧ����CO2��˵�������Ȼ���ͬʱ������ԭ�ӣ�Ȼ�ɶ���Ķ�Ԫȡ���������Ķ�Ԫȡ�����������4��̼ԭ��ʱ����Ԫȡ�����ﹲ��8�֣�
��HOOCCHClCH2CH2CH3����HOOCCH2CHClCH2CH3����HOOCCH2CH2CHClCH3����HOOCCH2CH2CH2CH2Cl��
��CH2ClCH��COOH��CH2CH3����CH3CCl��COOH��CH2CH3����CH3CH��COOH��CHClCH3����CH3CH��COOH��CH2CH2Cl��������3��̼ԭ��ʱ����Ԫȡ�����ﹲ��4�֣���HOOCCHClCH��CH3��2����HOOCCH2CCl��CH3��2����HOOCCH2CH��CH2Cl��CH3����CH2ClC��COOH����CH3��2�����Է���������ͬ���칹��Ϊ12�֣���4������ʽΪC3H6O2����״�л���˴Ź��������Ϸ����Ŀ��ǿ�ȱȷֱ�Ϊ:��3��3����3��2��1;��3��1��1��1 ��2��2��1��1�������ǿ��ܵĽṹ��ʽ����Ϊ CH3COOCH3�� CH3CH2COOH��HCOOCH2CH3��CH3�DO�DCH2CHO��CH3CH(OH)CHO��HOCH2CH2CHO����5���������ᣨ![]() ���ж��������ͬ���칹�壬��������FeCl3��Һ������ɫ��Ӧ��˵�����з��ǻ����ұ�������2��һ����ȡ���˵���Ƕ�λȡ���������������ͬ���칹�������
���ж��������ͬ���칹�壬��������FeCl3��Һ������ɫ��Ӧ��˵�����з��ǻ����ұ�������2��һ����ȡ���˵���Ƕ�λȡ���������������ͬ���칹�������![]() ����6�����л�������������ϵͳ������������2��2��4��5���ļ���3��3�����һ����顣
����6�����л�������������ϵͳ������������2��2��4��5���ļ���3��3�����һ����顣

����Ŀ�����¶�Ϊ20�棬Ũ��Ϊ1.0mol��L��1��H2SO4��2.2mol��L��1�ļ���Һ��50ml���(��Һ�ܶȾ�Ϊ1g��ml��1��������Ϊ4.184kJ��K��1��kg��1)�������������������Һ���¶ȱ仯�������£�
��Ӧ�� | ��ʼ�¶�t1�� | ��ֹ�¶�t2�� |
H2SO4+NaOH | 20 | 33.6 |
H2SO4+NH3��H2O | 20 | 32.6 |
��ӦNH3��H2O ��l��![]() NH4+(aq)+OH��(aq)���ʱ�ԼΪ(��λ��kJ��mol��1) �� ��
NH4+(aq)+OH��(aq)���ʱ�ԼΪ(��λ��kJ��mol��1) �� ��
A. +2.1 B. +4.2 C. +52.7 D. ȱ������������

