��Ŀ����
11�������仯������������������;�㷺����1����ѧʽΪMg17Al12�ĺϽ���һ������������������䴢���ԭ��Ϊ��Mg17Al12+17H2�T17MgH2+12Al
�õ��Ļ����X��17MgH2+12Al����һ�����������ͷų�H2
��MgH2����Ԫ�صĻ��ϼ�Ϊ-1
��1molX��������NaOH��Һ��Ӧ�ɵõ�35molH2
��2����ˮAlCl3�������л��ϳɵĴ�������ҵ�ϳ���Al2O3��Cl2�ͽ�̿�ڸ��������·�Ӧ�Ʊ���ˮAlCl3��ͬʱ����һ�ֿ�ȼ�����壬�÷�Ӧ�Ļ�ѧ����ʽ��Al2O3+3Cl2+3C�T2AlCl3+3CO
��3���ۺ��Ȼ�������PAFC���Ǹ�Ч��ˮ������I��ɿɱ�ʾΪ[AlFe��OH��nCl6-n]m�ۺ��Ȼ������ܷ�����ͼ��ʾ�ı仯��
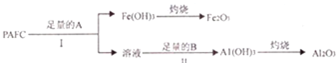
��PAFC��ǿ������Һ�лή�ͻ�ʧȥ��ˮ���ã���ԭ����ǿ���Ժ�ǿ������Һ�����������������������γ���Ӧ����
�ڲ�����з�Ӧ�����ӷ���ʽΪAlO2-+CO2+2H2O=Al��OH��3��+HCO3-��
���� ��1���ٸ���þΪ+2�ۼ��仯����Ļ��ϼ۴�����Ϊ0����MgH2����Ԫ�صĻ��ϼۣ�
�ڸ��ݻ����X��17MgH2+12Al����������NaOH��Һ��ϣ������������Ʒ�Ӧ�����������������ݴ˼��㣻
��2�����ݹ�ҵ�Ͽ���Al2O3��Cl2����̿��Ϸ�Ӧ�Ʊ���ˮAlCl3���÷�Ӧ����һ�ֲ����ǿ�ȼ���������ԭ���غ���ΪCO��д����ʽ��
��3����������������������������ˮ�з���ˮ�������������������������Ľ�������ˮ��������������ˮ������
��Ϊ���PAFC��Al��Fe������PAFC��������AΪ����������Һ��Ӧ������������������ƫ��������Һ���˺�õ������������յõ�����������Һ��ͨ�����������̼�����ѹ���������������������յõ����������Լ�AΪ����������Һ��������еķ�ӦΪƫ��������Һ��ͨ�����������̼����������������������
��� �⣺��1������ΪþΪ+2�ۣ��ֻ�����Ļ��ϼ۴�����Ϊ0������MgH2����Ԫ�صĻ��ϼ�Ϊ-1�ۣ��ʴ�Ϊ��-1��
�ڻ����X��17MgH2+12Al����������NaOH��Һ��ϣ������������Ʒ�Ӧ�������������䷽��ʽΪ��2Al+2NaOH+2H2O�T2NaAlO2+3H2������1molX�к��������ʵ���Ϊ12mol��������������Ϊ$\frac{12}{2}$��3mol=18mol����1molX�к�MgH2�����ʵ���Ϊ1��17mol=17mol������һ���������ɣ�18+17��=35molH2���ʴ�Ϊ��35��
��2����Ϊ��ҵ�Ͽ���Al2O3��Cl2����̿��Ϸ�Ӧ�Ʊ���ˮAlCl3���÷�Ӧ����һ�ֲ����ǿ�ȼ���������ԭ���غ���ΪCO�����Է���ʽΪAl2O3+3Cl2+3C�T2AlCl3+3CO���ʴ�Ϊ��Al2O3+3Cl2+3C�T2AlCl3+3CO��
��3������Ϊ��������������������ˮ�з���ˮ�������������������������Ľ�������ˮ��������������ˮ����ǿ���Ժ�ǿ������Һ��������������������������ˮ��������Ӧ���壬���Ծ���ʧȥ��ˮ���ã�
�ʴ�Ϊ��ǿ���Ժ�ǿ������Һ�����������������������γ���Ӧ���壻
��Ϊ���PAFC��Al��Fe������PAFC��������AΪ����������Һ��Ӧ������������������ƫ��������Һ���˺�õ������������յõ�����������Һ��ͨ�����������̼�����ѹ���������������������յõ��������������Լ�AΪ����������Һ��������еķ�ӦΪƫ��������Һ��ͨ�����������̼������������������������Ӧ�����ӷ���ʽΪ��AlO2-+CO2+2H2O=Al��OH��3��+HCO3-��
�ʴ�Ϊ��AlO2-+CO2+2H2O=Al��OH��3��+HCO3-��
���� ���⿼���������仯�������������������м���㷺����;����Ҫ���������ʵ�����Ӧ�ã��Ѷȣ�3���������̷������������������ǽ���ؼ�����Ŀ�Ѷ��еȣ�

 ��ʦ����ָ���ο�ʱϵ�д�
��ʦ����ָ���ο�ʱϵ�д�| A�� | �ȵĴ�����Һ����ȥ���;��ϵ����� | |
| B�� | �������ƿ�����������ߵĹ����� | |
| C�� | ��ҵ���õ�ⱥ��ʳ��ˮ��ý����� | |
| D�� | ����������ҽ�õ�θ���кͼ���һ�� |
| A�� | Fe | B�� | Al��OH��3 | C�� | NO | D�� | H2SO3 |
 ��һ�ȴ����ͬ���칹���У�������
��һ�ȴ����ͬ���칹���У�������| A�� | 1�� | B�� | 2�� | C�� | 3�� | D�� | 4�� |
| A�� | 3d | B�� | 2p | C�� | 5f | D�� | 4s |
| A�� | 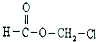 | B�� | 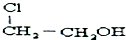 | C�� | CH2ClCHO | D�� | HOCH2CH2OH |
| ���� | HCOOH | HCN | H2CO3 |
| ����ƽ�ⳣ�� ��25�棩 | Ka=1.77��10-4 | Ka=4.9��10-10 | Ka1=4.3��10-7 Ka2=5.6��10-11 |
| A�� | 2CN-+H2O+CO2=2HCN+CO32- | |
| B�� | �к͵��������pH��HCOOH��HCN����NaOH����ǰ��С�ں��� | |
| C�� | ���ʵ���Ũ����ȵ�HCOONa��KCN��Һ�У�c��HCOOH��+c��HCOO-��=c��CN-��+c��HCN�� | |
| D�� | c��NH4+����ȵ�HCOONH4��Һ��NH4CN��Һ�У�c��NH4CN����c��HCOONH4�� |
 �������Ų�ʽ1s22s22p63s23p63d14s2����Ԫ�ص�ԭ������Ϊ21����Ԫ���ǽ���Ԫ�أ���������ǽ����������γɵĵ���Ϊ�������壮
�������Ų�ʽ1s22s22p63s23p63d14s2����Ԫ�ص�ԭ������Ϊ21����Ԫ���ǽ���Ԫ�أ���������ǽ����������γɵĵ���Ϊ�������壮