��Ŀ����
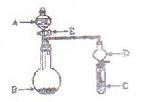
��15�֣���ͼ��ʾװ�ÿ�������ȡFe(OH)2�۲�Fe(OH)2�ڿ����б�����ʱ����ɫ�仯��ʵ��ʱ����ʹ����м��6 mol/L������Һ�������Լ���ѡ����д���пհף�
��1��B��ʢ��һ������NaOH��Һ��D���������� ����ʵ��D��������Ҫ
���� ��Һ��A��ӦԤ�ȼ�����Լ��� ��A�з�Ӧ�����ӷ���ʽ
Ϊ ��
��2��ʵ�鿪ʼʱӦ�Ƚ�����E ������رա����� ��C���յ���������ҪΪ ����Cƿ��Aƿ�е����� ʱ��������E �����
�رա������˿�Bƿ�п��ܷ����Ļ�ѧ��Ӧ�������ӷ���ʽ��ʾ���ǣ�
_ ��
��3����ȥװ��B�е���Ƥ����ʹ�������룬д���йط�Ӧ�Ļ�ѧ����ʽ��
��

��1��B��ʢ��һ������NaOH��Һ��D���������� ����ʵ��D��������Ҫ
���� ��Һ��A��ӦԤ�ȼ�����Լ��� ��A�з�Ӧ�����ӷ���ʽ
Ϊ ��
��2��ʵ�鿪ʼʱӦ�Ƚ�����E ������رա����� ��C���յ���������ҪΪ ����Cƿ��Aƿ�е����� ʱ��������E �����
�رա������˿�Bƿ�п��ܷ����Ļ�ѧ��Ӧ�������ӷ���ʽ��ʾ���ǣ�
_ ��
��3����ȥװ��B�е���Ƥ����ʹ�������룬д���йط�Ӧ�Ļ�ѧ����ʽ��
��
��1����Һ©�� ��6 mol/LH2SO4�� ���� �� Fe + 2H+ = Fe2+ + H2��
(2) �� ��H2 �� ���� �� �ر� ��H+ + OH�� = H2O �� Fe2+ + 2OH�� = Fe(OH)2��
(3) 4 Fe(OH)2 + O2 + 2H2O =" 4" Fe(OH)3
(2) �� ��H2 �� ���� �� �ر� ��H+ + OH�� = H2O �� Fe2+ + 2OH�� = Fe(OH)2��
(3) 4 Fe(OH)2 + O2 + 2H2O =" 4" Fe(OH)3
��1��D�����������Ƿ�Һ©������ʵ��D��������Ҫ����6 mol/LH2SO4��A��ӦԤ�ȼ�����Լ������ۣ�A�з�Ӧ�����ӷ���ʽ��Fe + 2H+ = Fe2+ + H2��
(2)ʵ�鿪ʼʱӦ�Ƚ�����E����C���յ���������ҪΪH2����Cƿ��Aƿ�е����ݼ���ʱ��������E���˿�Bƿ�п��ܷ����Ļ�ѧ��Ӧ�ǣ�H+ + OH�� =H2O �� Fe2+ + 2OH�� = Fe(OH)2��
��3����ȥװ��B�е���Ƥ����ʹ�������룬�йط�Ӧ�Ļ�ѧ����ʽ��4 Fe(OH)2 + O2 + 2H2O ="4" Fe(OH)3
(2)ʵ�鿪ʼʱӦ�Ƚ�����E����C���յ���������ҪΪH2����Cƿ��Aƿ�е����ݼ���ʱ��������E���˿�Bƿ�п��ܷ����Ļ�ѧ��Ӧ�ǣ�H+ + OH�� =H2O �� Fe2+ + 2OH�� = Fe(OH)2��
��3����ȥװ��B�е���Ƥ����ʹ�������룬�йط�Ӧ�Ļ�ѧ����ʽ��4 Fe(OH)2 + O2 + 2H2O ="4" Fe(OH)3

��ϰ��ϵ�д�
�����Ŀ
 ��100�G��
��100�G��

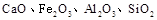 ��ɣ�����ʯ��ʯ��ȡ�Ȼ��ƺ���������ʵ�鲽�����£�
��ɣ�����ʯ��ʯ��ȡ�Ȼ��ƺ���������ʵ�鲽�����£�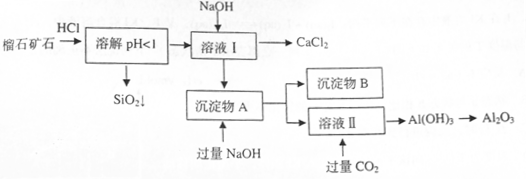
 �⣬�����еĽ��������� ��
�⣬�����еĽ��������� ��
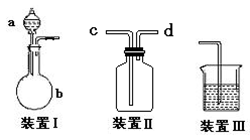
 ���岢ͨ����ҺII�У����û�г������������ܵ�ԭ�� ��Ϊ���ܲ���������ͬѧ��ͼIװ�ý����˸Ľ����Ľ��ķ���Ϊ ��
���岢ͨ����ҺII�У����û�г������������ܵ�ԭ�� ��Ϊ���ܲ���������ͬѧ��ͼIװ�ý����˸Ľ����Ľ��ķ���Ϊ ��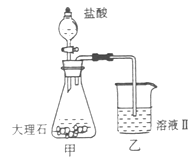
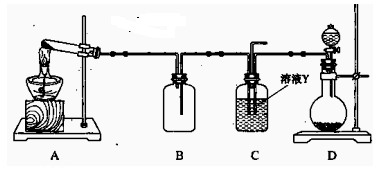

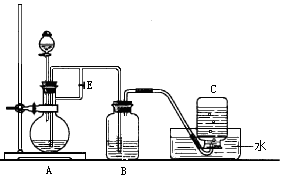
 ���������ʵ�顣��ʵ��װ������ͼ��ʾ��
���������ʵ�顣��ʵ��װ������ͼ��ʾ��