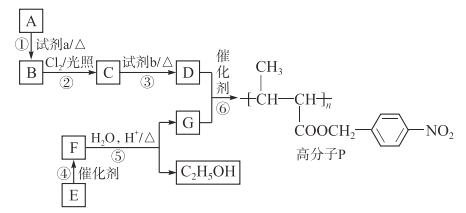
ΧβΡΩΡΎ»ί
ΓΨΧβΡΩΓΩΆ≠ «÷Ί“ΣΒΡΫπ τ≤ΡΝœΓΘ
(1)ΙΛ“Β…œΩ…”ΟCu2SΚΆO2Ζ¥”Π÷Τ»Γ¥÷Ά≠Θ§ΗΟΖ¥”Π÷–―θΜ·ΦΝΈΣ________ΓΘΒγΫβ¥÷Ά≠÷Τ»ΓΨΪΆ≠Θ§ΒγΫβ ±Θ§―τΦΪ≤ΡΝœ «________Θ§ΒγΫβ“Κ÷–±Ί–κΚ§”–ΒΡ―τάκΉ” «________ΓΘ
(2)‘Ύ100 mL 18 molΓΛLΘ≠1≈®ΝρΥα÷–Φ”»κΙΐΝΩΒΡΆ≠Τ§Θ§Φ”»» Ι÷°≥δΖ÷Ζ¥”ΠΘ§Ζ¥”Π÷–±ΜΜΙ‘≠ΒΡH2SO4ΈΣ________molΓΘ
(3)ΒγΉ”ΙΛ“Β‘χ”Ο÷ ΝΩΖ÷ ΐΈΣ30%ΒΡFeCl3»ή“ΚΗ· ¥Ζσ”–Ά≠≤≠ΒΡΨχ‘ΒΑε÷Τ”ΓΥΔΒγ¬ΖΑεΘ§ΈΣΝΥ¥” Ι”ΟΙΐΒΡΖœΗ· ¥“Κ÷–ΜΊ ’Ά≠Θ§≤Δ÷Ί–¬ΒΟΒΫFeCl3»ή“ΚΘ§…ηΦΤ»γœ¬ Β―ιΝς≥ΧΓΘ
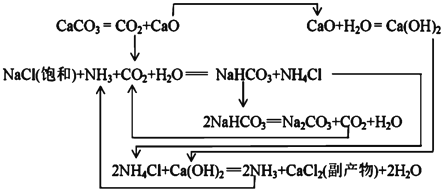
…œ ωΝς≥Χ÷–Θ§ΥυΦ” ‘ΦΝΒΡΜ·―ß ΫΈΣΘΚX________Θ§Y________Θ§Z________ΘΜΒΎΔό≤ΫΖ¥”ΠΒΡάκΉ”ΖΫ≥Χ ΫΈΣ___________________ΓΘ
ΓΨ¥πΑΗΓΩ(1) Cu2SΚΆO2 ¥÷Ά≠ Cu2ΘΪ –Γ”Ύ0.9 Fe HCl Cl2 2Fe2ΘΪΘΪCl2=2Fe3ΘΪΘΪ2ClΘ≠
ΓΨΫβΈωΓΩ
Θ®1Θ©ΙΛ“Β…œΩ…”ΟCu2SΚΆO2Ζ¥”Π÷Τ»Γ¥÷Ά≠ ±Θ§Ά≠‘ΣΥΊΚΆ―θ‘ΣΥΊΜ·ΚœΦέΫΒΒΆ±ΜΜΙ‘≠Θ§Cu2SΚΆO2Ήω―θΜ·ΦΝΘΜΒγΫβ¥÷Ά≠÷Τ»ΓΨΪΆ≠ ±Θ§¥÷Ά≠Ήω―τΦΪΘ§ΨΪΆ≠Ήω“θΦΪΘ§Κ§”–Ω…»ή–‘Ά≠―ΈΒΡ»ή“ΚΉωΒγΫβ÷ »ή“ΚΘ§Ι ¥πΑΗΈΣΘΚCu2SΚΆO2ΘΜ¥÷Ά≠ΘΜCu2ΘΪΘΜ
Θ®2Θ©Ά≠ΚΆ≈®ΝρΥαΙ≤»»Ζ¥”Π ±Θ§≈®ΝρΥα‘ΎΖ¥”Π÷–ΤπΒΫ―θΜ·ΦΝΚΆΥαΒΡΉς”ΟΘ§ΥφΉ≈Ζ¥”ΠΫχ––Θ§ΝρΥα≈®Ε»Φθ–ΓΘ§Ά≠”κœΓΝρΥα≤ΜΖ¥”ΠΘ§‘ρ‘Ύ100 mL 18 molΓΛLΘ≠1≈®ΝρΥα÷–Φ”»κΙΐΝΩΒΡΆ≠Τ§Θ§Φ”»» Ι÷°≥δΖ÷Ζ¥”ΠΘ§Ζ¥”Π÷–±ΜΜΙ‘≠ΒΡH2SO4–Γ”Ύ0.9Θ§Ι ¥πΑΗΈΣΘΚ–Γ”Ύ0.9ΘΜ
Θ®3Θ©”Ο30%ΒΡFeCl3»ή“ΚΗ· ¥”–Ά≠≤≠ΒΡΨχ‘ΒΑε”ΓΥΔΒγ¬ΖΑε ±ΖΔ…ζΖ¥”ΠΘΚ2Fe3ΘΪΘΪCu=2Fe2ΘΪΘΪCu2ΘΪΘ§Ζœ“Κ÷–ΜαΚ§”–Fe2ΘΪΓΔCu2ΘΪΓΔFe3ΘΪΘΜΦ”»κΧζΖέΚσΘ§2Fe3ΘΪΘΪFe=3Fe2ΘΪΓΔFeΘΪCu2ΘΪ=CuΘΪFe2ΘΪΘΜ¬Υ‘ϋ÷–Κ§”–ΙΐΝΩΒΡFeΚΆCuΘ§Φ”»κ―ΈΥαΘΚFeΘΪ2HCl=FeCl2ΘΪH2ΓϋΘΜΆ®»κCl2ΚσΘ§2FeCl2ΘΪCl2=2FeCl3Θ§Ι ¥πΑΗΈΣΘΚFeΘΜHClΘΜCl2ΘΜ2Fe2ΘΪΘΪCl2=2Fe3ΘΪΘΪ2ClΘ≠ΓΘ

 ΧΊΗΏΦΕΫΧ ΠΒψ≤ΠœΒΝ–¥πΑΗ
ΧΊΗΏΦΕΫΧ ΠΒψ≤ΠœΒΝ–¥πΑΗΓΨΧβΡΩΓΩΧΦΥαΡΤΒΡ”ΟΆΨΚήΙψΘ§Ω…”ΟΉω“±ΫπΓΔΖΡ÷·ΓΔΤ·»ΨΒ»ΙΛ“ΒΒΡΜυ±Ψ‘≠ΝœΘ°«κΗυΨίΧβ“βΜΊ¥πœ¬Ν–Έ ΧβΘΚ
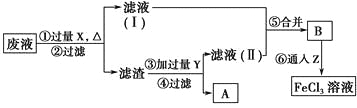
Δώ![]() άΫγΉν‘γΙΛ“Β…ζ≤ζΧΦΥαΡΤΒΡΖΫΖ® «¬Ζ≤ΦάΦ
άΫγΉν‘γΙΛ“Β…ζ≤ζΧΦΥαΡΤΒΡΖΫΖ® «¬Ζ≤ΦάΦ![]() Ζ®Θ°ΤδΝς≥Χ»γœ¬ΘΚ
Ζ®Θ°ΤδΝς≥Χ»γœ¬ΘΚ

![]() Νς≥ΧIΒΡΝμ“Μ≤ζΈο « ______ Θ§Νς≥ΧΔρΒΡΖ¥”ΠΖ÷≤ΫΫχ––ΘΚ
Νς≥ΧIΒΡΝμ“Μ≤ζΈο « ______ Θ§Νς≥ΧΔρΒΡΖ¥”ΠΖ÷≤ΫΫχ––ΘΚ![]()
![]() ΘΜ
ΘΜ
![]() ”κ ·Μ“ ·ΖΔ…ζΗ¥Ζ÷ΫβΖ¥”ΠΘ§ΉήΖ¥”ΠΖΫ≥Χ ΫΩ…±μ ΨΈΣ ______ Θ°
”κ ·Μ“ ·ΖΔ…ζΗ¥Ζ÷ΫβΖ¥”ΠΘ§ΉήΖ¥”ΠΖΫ≥Χ ΫΩ…±μ ΨΈΣ ______ Θ°
Δρ![]() ΡξΘ§±»άϊ ±»ΥΥςΕϊΈ§
ΡξΘ§±»άϊ ±»ΥΥςΕϊΈ§![]()
![]() ”ΟΑ±ΦνΖ®…ζ≤ζΧΦΥαΡΤΘ°Ζ¥”Π‘≠άμ»γœ¬ΘΚ
”ΟΑ±ΦνΖ®…ζ≤ζΧΦΥαΡΤΘ°Ζ¥”Π‘≠άμ»γœ¬ΘΚ
![]() ±“Μ–©Έο÷ ‘ΎΥ°÷–ΒΡ»ήΫβΕ»
±“Μ–©Έο÷ ‘ΎΥ°÷–ΒΡ»ήΫβΕ»![]()
NaCl |
|
|
|
|
|
|
|
|
|
![]() Α±ΦνΖ®…ζ≥…¥ΩΦνΒΡ‘≠Νœ « ______ Θ§Ω…―≠ΜΖάϊ”ΟΒΡΈο÷ ”– ______ Θ°
Α±ΦνΖ®…ζ≥…¥ΩΦνΒΡ‘≠Νœ « ______ Θ§Ω…―≠ΜΖάϊ”ΟΒΡΈο÷ ”– ______ Θ°
![]() ±ΞΚΆNaCl»ή“ΚΆ®
±ΞΚΆNaCl»ή“ΚΆ®![]() ΚΆ
ΚΆ![]() Ρή…ζ≥…
Ρή…ζ≥…![]() ΒΡ‘≠“ρ”–ΘΚ ______ Θ°
ΒΡ‘≠“ρ”–ΘΚ ______ Θ°
Δσ![]() Έ“ΙζΜ·ΙΛΉ®Φ“ΚνΒ¬Αώ―–ΨΩ≥ωΝΣΚœ÷ΤΦνΖ®Θ§ΤδΖ¥”Π‘≠άμΚΆΑ±ΦνΖ®άύΥΤΘ§ΒΪΫΪ÷ΤΑ±ΚΆ÷ΤΦνΝΣΚœΘ§ΧαΗΏΝΥ‘≠Νœάϊ”Ο¬ Θ°
Έ“ΙζΜ·ΙΛΉ®Φ“ΚνΒ¬Αώ―–ΨΩ≥ωΝΣΚœ÷ΤΦνΖ®Θ§ΤδΖ¥”Π‘≠άμΚΆΑ±ΦνΖ®άύΥΤΘ§ΒΪΫΪ÷ΤΑ±ΚΆ÷ΤΦνΝΣΚœΘ§ΧαΗΏΝΥ‘≠Νœάϊ”Ο¬ Θ°

![]() …ζ≤ζ÷––ηœρΖ÷άκ≥ω
…ζ≤ζ÷––ηœρΖ÷άκ≥ω![]() ΚσΥυΒΟΒΡ»ή“Κ÷–Φ”»κNaClΙΧΧε≤ΔΆ®»κ
ΚσΥυΒΟΒΡ»ή“Κ÷–Φ”»κNaClΙΧΧε≤ΔΆ®»κ![]() Θ§‘Ύ ______
Θ§‘Ύ ______ ![]() ΧνΈ¬Ε»ΖΕΈß
ΧνΈ¬Ε»ΖΕΈß![]() œ¬Έω≥ω ______
œ¬Έω≥ω ______ ![]() ΧνΜ·―ß Ϋ
ΧνΜ·―ß Ϋ![]()
ΓΨΧβΡΩΓΩάϊ”Ο¥ΏΜ·ΦΦ θΩ…ΫΪΤϊ≥ΒΈ≤Τχ÷–ΒΡNOΚΆCOΉΣ±δ≥…![]() ΚΆ
ΚΆ![]() Θ§Μ·―ßΖΫ≥Χ Ϋ»γœ¬ΘΚ
Θ§Μ·―ßΖΫ≥Χ Ϋ»γœ¬ΘΚ![]() Ρ≥Έ¬Ε»œ¬Θ§‘Ύ»ίΜΐ≤Μ±δΒΡΟή±’»ίΤς÷–Ά®»κNOΚΆCOΘ§≤βΒΟ≤ΜΆ§ ±ΦδΒΡNOΚΆCOΒΡ≈®Ε»»γœ¬±μΘΚœ¬Ν–ΥΒΖ®÷–Θ§≤Μ’ΐ»ΖΒΡ «
Ρ≥Έ¬Ε»œ¬Θ§‘Ύ»ίΜΐ≤Μ±δΒΡΟή±’»ίΤς÷–Ά®»κNOΚΆCOΘ§≤βΒΟ≤ΜΆ§ ±ΦδΒΡNOΚΆCOΒΡ≈®Ε»»γœ¬±μΘΚœ¬Ν–ΥΒΖ®÷–Θ§≤Μ’ΐ»ΖΒΡ «
±Φδ | 0 | 1 | 2 | 3 | 4 | 5 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
A.2sΡΎΒΡΤΫΨυΖ¥”ΠΥΌ¬ ![]()
B.‘ΎΗΟΈ¬Ε»œ¬Θ§Ζ¥”ΠΒΡΤΫΚβ≥Θ ΐ![]()
C.»τΫΪNOΚΆCOΒΡΤπ ΦΆΕΝœΝΩΨυΦ”±ΕΘ§‘ρΤΫΚβ ±NOΉΣΜ·¬ –Γ”Ύ![]()
D. Ι”Ο¥ΏΜ·ΦΝΩ…“‘ΧαΗΏΒΞΈΜ ±ΦδCOΚΆNOΒΡ¥ΠάμΝΩ