��Ŀ����
18��1mol�������ΪC3H8O��Һ̬�л���A���������Ľ��������ã�������11.2L��������״��������A�����б���һ���ǻ������˻���̼����һ�ˣ���A�Ľṹ��ʽΪCH3CH2CH2OH��A��Ũ���Ṳ�ȣ���������ȥ1����ˮ������B����Ӧ�ķ���ʽΪCH3CH2CH2OH$��_{��}^{ŨH_{2}SO_{4}}$CH3CH=CH2+H2O��Bͨ����ˮ�ܷ����ӳɷ�Ӧ����Ӧ�ķ���ʽΪCH3CH=CH2+Br2��CH3CHBr-CH2Br������ n��H2��=$\frac{11.2L}{22.4L/mol}$=0.5mol�����л�����������ǻ���̼����һ�ˣ�ӦΪCH3CH2CH2OH����Ũ���������¿�����CH3CH=CH2������ˮ�����ӳɷ�Ӧ������CH3CHBrCH2Br��
��� �⣺1molC3H8O��Na��Ӧ����n��H2��=$\frac{11.2L}{22.4L/mol}$=0.5mol����C3H8O����1���ǻ������ǻ���̼����һ�ˣ���A����֧������AӦΪCH3CH2CH2OH��
��Ũ���������£�CH3CH2CH2OH������ȥ��Ӧ����BΪCH3CH=CH2����Ӧ����ʽΪ��CH3CH2CH2OH$��_{��}^{ŨH_{2}SO_{4}}$CH3CH=CH2+H2O��CH3CH=CH2����ˮ�����ӳɷ�Ӧ����CH3CHBrCH2Br����Ӧ����ʽΪ��CH3CH=CH2+Br2��CH3CHBr-CH2Br��
�ʴ�Ϊ���ǣ�CH3CH2CH2OH��CH3CH2CH2OH$��_{��}^{ŨH_{2}SO_{4}}$CH3CH=CH2+H2O���ӳɣ�CH3CH=CH2+Br2��CH3CHBr-CH2Br��
���� ���⿼���л�����ƶϡ������Žṹ�����ʡ��л���Ӧ���͡��л���Ӧ����ʽ��д�ȣ���Ŀ�ѶȲ���ע��Ի���֪ʶ���������գ�

 �ǻۿ����ܾ�100�ֵ�Ԫ���ؼ��ϵ�д�
�ǻۿ����ܾ�100�ֵ�Ԫ���ؼ��ϵ�д� ��Ԫ������ĩ��ϵ�д�
��Ԫ������ĩ��ϵ�д�| A�� | ��֪I2������KI�γ�KI3������֧ʢ��KI3��Һ���Թ��У��ֱ�μӵ�����Һ��AgNO3��Һ��ǰ����Һ�����������л�ɫ������˵��KI3��Һ�д���ƽ�⣺I3-?I2+I- | |
| B�� | ���ʵ���Ũ����ȵ�H2CO3��Na2CO3��Һ�������Ϻ����Һ��c��Na+����c��HCO3-����c��CO32-����c��OH-����c��H+�� | |
| C�� | ��֪��Ksp��AgCl��=1.8��10-10��Ksp��Ag2CrO4��=2.0��10-12�����������Ũ��Ϊ1.0��10-4mol/L��AgNO3��Һ���뵽Ũ�Ⱦ�Ϊ1.0��10-4mol/L��KCl��K2CrO4�Ļ����Һ�в������ֲ�ͬ��������Ag2CrO4�����Ȳ��� | |
| D�� | 25��ʱ����Һ��ˮ�������c��H+����ˮ�������c��OH-���ij˻�һ������10-14 |
���и���ʵ������ó��Ľ�����ȷ����
ѡ�� | ʵ����� | ���� | ���� |
A | ��SO2ͨ������KMnO4��Һ�� | ��ɫ��ȥ | SO2����Ư���� |
B | C2H5OH��Ũ�����Ϻ���ȵ�170�� | ���ɵ�����ʹ����KMnO4��Һ��ɫ | ��Ӧһ����������ϩ |
C | ��KNO3��KOH�����Һ�м������۲����ȣ��ܿڷ�ʪ��ĺ�ɫʯ�� | ��ֽ��Ϊ��ɫ | NO3-����ԭΪNH3 |
D | ��NaSiO3��Һ�еμ�I�η�̪��Һ��Ȼ����μ���ϡ��������ɫ��ȥ | ������װ���� | �ǽ����ԣ�Cl��Si |
| A�� | 2�� | B�� | 3�� | C�� | 4�� | D�� | 5�� |
| A�� | �ӳɡ���ȥ��ȡ�� | B�� | ��ȥ���ӳɡ�ȡ�� | C�� | ȡ������ȥ���ӳ� | D�� | ��ȥ���ӳɡ���ȥ |

| A�� | ������Ӧʱ�������ʵķ�Ӧ���ʴ�С��ϵΪv��X��=v ��Y��=2 v ��Z�� | |
| B�� | ͼa �з�Ӧ�ﵽƽ��ʱ��Y ��ת����Ϊ37.5% | |
| C�� | T0 ʱ���÷�Ӧ��ƽ�ⳣ��Ϊ 33.3 | |
| D�� | �÷�Ӧ����Ӧ�ķ�Ӧ�ȡ�H��0 |
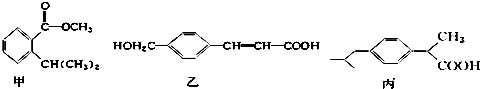
�����й�˵���д�����ǣ�������
| A�� | �ס��ҡ������Ƿ����廯���ֻ����������̼��������Һ��Ӧ | |
| B�� | ֻ��̼��������Һ��������Һ�ܼ���ס��ҡ��� | |
| C�� | ������������Ӧ����ͬ���ʵ����ļס��ҡ����������������ʵ���֮��Ϊ3��4��3 | |
| D�� | �Ļ�ѧʽΪC11H14O2���Һ������ֺ��������� |
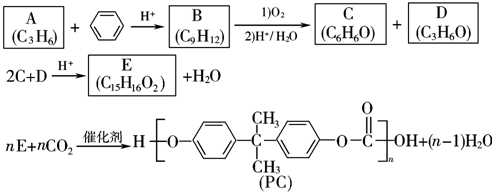
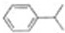 ��
��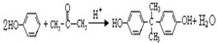 ��
��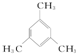 ��д���ṹ��ʽ����
��д���ṹ��ʽ����