��Ŀ����
ij���Ӿ��徧���ṹ��ͼ1��ʾ��Xλ��������Ķ��㣬Yλ�����������ģ��Է�����
��1��������ÿ��Yͬʱ������ ��X��ÿ��Xͬʱ������ ��Y���þ���Ļ�ѧʽΪ ��
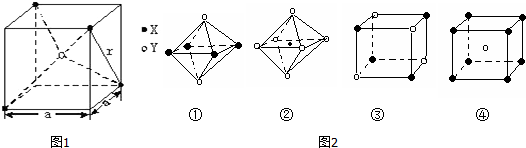
��2��������ÿ��X��Χ������ӽ��Ҿ�����ȵ�X���� ����
��3�������о��������2��X��1��Y�γɵļнǡ�XYX�Ķ���Ϊ ��
��4����þ����Ħ������ΪM g?mol-1��������ܶ�Ϊ�� g?cm-3�������ӵ�����ΪNA���������������������X���ļ�ľ���Ϊ cm��
��5��ͼ2��ͼ���Ǵ�NaCl��CsCl����ṹͼ�зָ�����IJ��ֽṹͼ�����ж�NaCl����ṹ��ͼ���� ��
��1��������ÿ��Yͬʱ������

��2��������ÿ��X��Χ������ӽ��Ҿ�����ȵ�X����
��3�������о��������2��X��1��Y�γɵļнǡ�XYX�Ķ���Ϊ
��4����þ����Ħ������ΪM g?mol-1��������ܶ�Ϊ�� g?cm-3�������ӵ�����ΪNA���������������������X���ļ�ľ���Ϊ
��5��ͼ2��ͼ���Ǵ�NaCl��CsCl����ṹͼ�зָ�����IJ��ֽṹͼ�����ж�NaCl����ṹ��ͼ����
���㣺�����ļ���
ר�⣺��ѧ���뾧��ṹ
��������1��������ÿ��Yͬʱ������4��X��ÿ��Xͬʱ������8��Y�����þ�̯��ȷ����ѧʽ��
��2��������ÿ��X��Χ������ӽ��Ҿ�����ȵ�X����=3��8��
��
��3���þ������ĸ�X��һ��Yԭ���γ���������ṹ��
��4���辧�����������������X���ļ�ľ���Ϊacm�������ⳤ=
acm�����=
cm3�����ݦ�=
���㣻
��5��NaCl�����������壬ÿ����������Χ��6�������ӡ�ÿ����������Χ��6�������ӣ�
��2��������ÿ��X��Χ������ӽ��Ҿ�����ȵ�X����=3��8��
| 1 |
| 2 |
��3���þ������ĸ�X��һ��Yԭ���γ���������ṹ��
��4���辧�����������������X���ļ�ľ���Ϊacm�������ⳤ=
| ||
| 2 |
| ||
| 4 |
| m |
| V |
��5��NaCl�����������壬ÿ����������Χ��6�������ӡ�ÿ����������Χ��6�������ӣ�
���
�⣺��1������ͼ��֪��������ÿ��Yͬʱ������4��X��ÿ��Xͬʱ������8��Y���þ�����Xԭ�Ӹ���=4��
=
��Yԭ�Ӹ���=1������X��Yԭ�Ӹ���֮��Ϊ1��2���仯ѧʽΪXY2��Y2X���ʴ�Ϊ��4��8��XY2��Y2X��
��2��������ÿ��X��Χ������ӽ��Ҿ�����ȵ�X����=3��8��
=12���ʴ�Ϊ��12��
��3���þ������ĸ�X��һ��Yԭ���γ���������ṹ�������������109��28�䣬�ʴ�Ϊ��109��28�䣻
��4���辧�����������������X���ļ�ľ���Ϊacm�������ⳤ=
acm������T
cm3����=
=
=��������a=
���ʴ�Ϊ��
��
��5��NaCl�����������壬ÿ����������Χ��6�������ӡ�ÿ����������Χ��6�������ӣ�������λ��֪�ڢ���ȷ���ʴ�Ϊ���ڢܣ�
| 1 |
| 8 |
| 1 |
| 2 |
��2��������ÿ��X��Χ������ӽ��Ҿ�����ȵ�X����=3��8��
| 1 |
| 2 |
��3���þ������ĸ�X��һ��Yԭ���γ���������ṹ�������������109��28�䣬�ʴ�Ϊ��109��28�䣻
��4���辧�����������������X���ļ�ľ���Ϊacm�������ⳤ=
| ||
| 2 |
| ||
| 4 |
| m |
| V |
| ||||
|
| 2 |
| 3 |
| ||
| 2 |
| 3 |
| ||
��5��NaCl�����������壬ÿ����������Χ��6�������ӡ�ÿ����������Χ��6�������ӣ�������λ��֪�ڢ���ȷ���ʴ�Ϊ���ڢܣ�
���������⿼���˾����ļ��㣬�����þ�̯�����㾧�����������ǽⱾ��ؼ����ٽ�ϻ�����ʽ����ѵ��Ǿ������ܶȡ���λ���ļ��㣬ͬʱ����ѧ���ռ�������������ѧ�����������Ѷ��еȣ�

��ϰ��ϵ�д�
�����Ŀ
�Ƚ�1mol������1molһ����̼��������������
�ٷ��ӵ����ʵ���
��ԭ�ӵ����ʵ���
������
�ܵ�����
����������������ͬ���� ��
�ٷ��ӵ����ʵ���
��ԭ�ӵ����ʵ���
������
�ܵ�����
����������������ͬ����
��֪������ͬ�۵���������ͬԭ�����ķ��ӻ����Ӿ�����ͬ�Ľṹ����һԭ����Ϊ���ȵ���ԭ���������ݵȵ���ԭ�������и������ӽṹ�����Ƶ��ǣ�������
| A��BCl3��PH3 |
| B��NH4+��CH4 |
| C��NO3-��CO32- |
| D��CO2��N2O |
���з�Ӧ��HNO3�ȱ��ֳ������ֱ��ֳ�ǿ�����Ե��ǣ�������
| A��ϡ������Fe2O3��Ӧ |
| B��ϡ������NaOH��Һ��Ӧ |
| C��Ũ���������ȵ�̼��Ӧ |
| D��Ũ������ͭ��Ӧ |