��Ŀ����
����Ŀ��A��B��C��X��Ϊ��ѧ�����Ĵ��������֮��������ת����ϵ����Ӧ����������������ȥ����
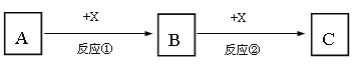
��1����A��B��C��Ϊ����ͬ�ַǽ���Ԫ�صĻ����AΪʹʪ��ĺ�ɫʯ����ֽ���������壬B�Ӵ��������̱�ΪC����Ӧ�ٵĻ�ѧ����ʽΪ_______________________��
��2����A��B��CΪ��ɫ��Ӧ���ʻ�ɫ�Ļ����XΪ��ɫ��ζ���壬��Ӧ�ڵ����ӷ���ʽΪ_____________________________________________��
��3����A��B��C��Ϊ����ͬ�ֽ���Ԫ�صĻ����X��ǿ���Ӧ�ڵ����ӷ���ʽΪ________________________________________________��
��4����AΪ����Fe��XΪϡ���ᣬ����B����Һ�м�������������Һ������Ϊ________________________________________��
��5����A��X��Ϊ���ʣ�BΪ��ʹƷ����Һ��ɫ�����壬��Ӧ�ڵĻ�ѧ����ʽΪ_______________________________________________________________��
���𰸡�4NH3+5O2![]() 4NO+6H2OCO32-+CO2+H2O��2HCO3-Al��OH��3+OH-��AlO2-+2H2O�Ȳ�����ɫ������Ѹ�ٱ�Ϊ����ɫ�����ձ�Ϊ���ɫ2SO2+O2
4NO+6H2OCO32-+CO2+H2O��2HCO3-Al��OH��3+OH-��AlO2-+2H2O�Ȳ�����ɫ������Ѹ�ٱ�Ϊ����ɫ�����ձ�Ϊ���ɫ2SO2+O2![]() 2SO3
2SO3
��������
��1����A��B��C��Ϊ����ͬ�ַǽ���Ԫ�صĻ����AΪʹʪ��ĺ�ɫʯ����ֽ���������壬ӦΪNH3��B�Ӵ��������̱�ΪC����֪XΪO2��BΪNO��CΪNO2����Ӧ�ٵĻ�ѧ����ʽΪ4NH3+5O2![]() 4NO+6H2O��
4NO+6H2O��
��2����A��B��CΪ��ɫ��Ӧ���ʻ�ɫ�Ļ����Ӧ����NaԪ�أ�XΪ��ɫ��ζ���壬ӦΪCO2����ת����ϵ��֪AΪNaOH��BΪNa2CO3��CΪNaHCO3����Ӧ�ڵ����ӷ���ʽΪCO32-+CO2+H2O��2HCO3-��
��3����A��B��C��Ϊ����ͬ�ֽ���Ԫ�صĻ����X��ǿ���֪���е�Ԫ��ΪAl��XΪNaOH��ǿ���A����Al3+��BΪAl��OH��3��C����AlO2-����Ӧ�ڵ����ӷ���ʽΪAl��OH��3+OH-��AlO2-+2H2O��
��4����AΪ����Fe��XΪϡ���ᣬBΪFe��NO3��2��CΪFe��NO3��3������B����Һ�м�������������Һ������Ϊ�Ȳ�����ɫ������Ѹ�ٱ�Ϊ����ɫ�����ձ�Ϊ���ɫ��
��5����A��X��Ϊ���ʣ�BΪ��ʹƷ����Һ��ɫ�����壬��BΪSO2��AΪS��XΪO2��CΪSO3����Ӧ�ڵĻ�ѧ����ʽΪ2SO2+O2![]() 2SO3��
2SO3��

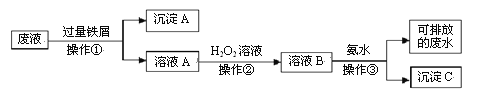
����Ŀ��ˮ�ܿ����Ҫ�ɷ�ΪCo2O3������SiO2������Al2O3��Fe2O3�� CuO��MnO2�ȡ�һ������ˮ�ܿ���ȡCoCl2��6H2O �Ĺ����������£�
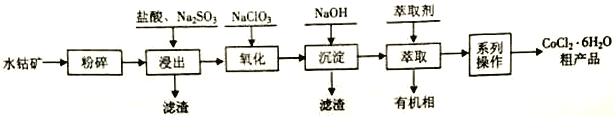
��֪�� ��CoCl2��6H2O������ʧȥ�ᾧˮ��
��25 ��ʱ���趨��Һ��ij�������ӳ�ʼŨ��Ϊ0.1 molL-1�����ֳ����IJο��������±�(��������ȫ��ָ��Һ�и�����Ũ�ȡ�1.0��10-5molL-1)��
���� | Al(OH)3 | Fe(OH)3 | Fe(OH)2 | Co(OH)2 | Cu(OH)2 | Mn(OH)2 |
��ʼ������pH | 3.4 | 1.5 | 6.3 | 7.0 | 4.7 | 8.1 |
������ȫʱpH | 4.7 | 2.8 | 8.3 | 9.0 | 6.7 | 10.1 |
�ش���������
��1������25��ʱCo(OH)2��Ksp =_______________��
��2�����������м���һ������Na2SO3��ԭCo2O3��MnO2�ȣ�Co2O3������Ӧ�����ӷ���ʽΪ_______________________________��
��3����������Ҫ����NaClO3�������������������NaClO3���������ɵ��ж�������______________������������Ҫ��Ӧ�����ӷ���ʽΪ_______________________________��
��4����֪�¶ȶ�ͭ���ܡ����Ľ����ʵ�Ӱ��������ͼ����ȡ��A��B��pH���ܡ���������ȡ�ʵ�Ӱ��������ͼ��

�ٽ����¶ȿ�����50-60���ԭ����_________________________��
��Ӧѡ����ȡ��_________________________���A����B������
��5����ϵ�в�����������____________��______________���˵ȣ��Ƶõ�CoCl2��6H2O���ѹ��ɵ�ԭ����_________________________��