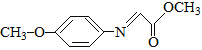
��Ŀ����
����Ŀ��ij�Ȼ�����Ʒ����FeCl2���ʡ���Ҫ�ⶨ������Ԫ�ص�����������ʵ�鰴���²�����У�
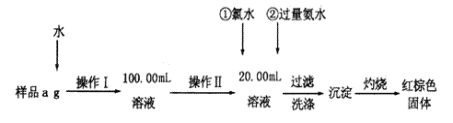
(1)����I���õ��IJ����������ձ�����Ͳ��100mL������ƿ�⣬��������_________(����������)������ƿʹ��ǰ������еIJ�����_________(�����)
A.���� B.��© C.��ʪ
(2)д��������ˮ������Ӧ�����ӷ���ʽ_______________________________________���÷�Ӧ��������ˮ�����������Լ��е�______________����(�����)��
A��H2O2 B����ˮ C��NaClO
(3)��������Ѿ�ϴ�Ӹɾ��IJ�����������__________________________��
(4)��������ΪW1g�����Ⱥ����������ɫ����������ΪW2g������Ʒ����Ԫ�ص�����������____________________(�г�ԭʼ��ʽ�����軯��)
���𰸡�����������ͷ�ι� B 2Fe 2++Cl2=2Fe3++2Cl- AC ȡ���һ��ϴ��Һ���Թܣ��μ����������ữ���ټ�����������Һ�����ް�ɫ�������ɣ���֤���Ѿ�ϴ�Ӹɾ� 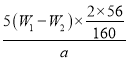 ��100%
��100%
��������
��ʵ��Ŀ���Dzⶨ����������������ȡ�ķ�����ʹ��Ʒ�ܽ⡢��Ӧ������������������Ȼ��ͨ������������������������������
(1)��ͼ��֪������I�ǽ���ˮ�ܽ�����Һϡ�ͳ�100.00mL��Һ����Ҫ�ձ��ܽ⣬�ò��������裬�����ȣ�������Ҫ��ͷ�ιܣ�����ƿʹ��ǰ��Ҫ��©��
(2)����ˮ������+2������Ϊ+3�ۣ�������ˮ�����ù������⡢�������ƴ���������������ˮ�еⵥ�ʲ��������������ӣ�
(3)��Һ�д����Ȼ�泥������������������Һ�������һ��ϴ��Һ���Ƿ���������ӣ����жϳ����Ƿ�ϴ����
(4)���ȷֽ����õ�������Fe2O3��������Ϊ(W2-W1)g������ʹ��20.00mL��Һ����ʵ�飬���100.00mL��Һ���Եõ�Fe2O3����Ϊ5(W2-W1)g�����ݻ�ѧʽ������Ԫ�ص����������������������Ķ������ԭ�Ȼ�����Ʒ����Ԫ�ص�����������
��1����ͼ��֪������I�ǽ���ˮ�ܽ�����Һϡ�ͳ�100.00mL��Һ��������������Ͳ��100mL������ƿ�⣬���������ܽ���ձ��Ͳ�����������ƿ��������̶���1~2cmʱ��Ҫ�ý�ͷ�ιܶ��ݣ�����ȱ�ٵ������Dz������ͽ�ͷ�ιܣ�����ƿʹ��ǰ������еIJ����Ǽ���ܷ�©ˮ����˺���ѡ����B��
(2)����ˮ����ʹ+2��Fe2+����Ϊ��Ϊ+3��Fe3+���÷�Ӧ�����ӷ���ʽΪ��2Fe 2++Cl2=2Fe3++2Cl-����������ˮ�����ù������⡢�������ƴ�������������������ˮ�еⵥ�ʲ��������������ӣ���˺���ѡ����AC��
(3)Fe(OH)3�����Ǵ�NH4Cl��Һ�й��˳����ģ�Fe��OH��3�������������Ȼ�泥������������������Һ�������һ��ϴ��Һ���Ƿ���������ӣ����鷽���ǣ�ȡ���һ��ϴ��Һ���Թ��У��μ�ϡ�����ữ���ټ���������������Һ�����ް�ɫ�������ɣ���֤����ϴ�Ӹɾ���
(4)����Ԫ�������غ㣬������ɫ�����е���������Ʒ������Fe2O3������ΪW2-W1g�����ڲμӷ�Ӧ����Һֻȡ������Һ��![]() �������Ԫ�ص�����Ϊ5(W2-W1)g��
�������Ԫ�ص�����Ϊ5(W2-W1)g��![]() ������Ʒ����Ԫ�ص�����������
������Ʒ����Ԫ�ص����������� ��100%��
��100%��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ��������(SO2Cl2)����������(SOCl2)�������Ȼ�������������ҽҩ��ũҩ��Ⱦ�Ϲ�ҵ���л��ϳɹ�ҵ���������Ȼ����������������(Li/SOCl2)��ء�
�й����ʵIJ����������±���
���� | �۵�/�� | �е�/�� | �������� |
SO2Cl2 | -54.1 | 69.1 | ����ˮ�⣬������������ ���ֽ⣺SO2Cl2 |
H2SO4 | 10.4 | 338 | ������ˮ�����ѷֽ� |
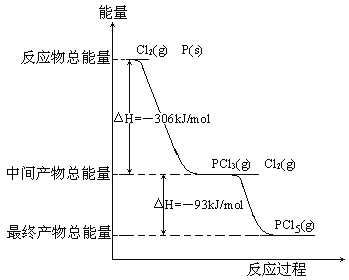
ʵ�����ø���������Ķ�������������ϳ������ȣ���Ӧ���Ȼ�ѧ����ʽΪ��SO2(g)+Cl2(g) ![]() SO2Cl2(l) ��H= - 97.3 kJ��mol-1����Ӧװ����ͼ��ʾ���г�������ʡ�ԣ�����ش��й����⣺
SO2Cl2(l) ��H= - 97.3 kJ��mol-1����Ӧװ����ͼ��ʾ���г�������ʡ�ԣ�����ش��й����⣺

��1������A������Ϊ___________��
��2������B��������_____________________��
��3��װ�ñ���ʢ�ŵ��Լ�Ϊ____________����ʵ�����������������ն��������ȷ�Ӧ�����ӷ���ʽΪ___________________��
��4��Ϊ��߱�ʵ���������ȵIJ��ʣ���ʵ���������Ҫע���������_________(�����)
����ͨ����ˮ����ͨ���� �ڿ����������ʣ��������˿�
����������ƿ���̣����ʵ����� �ܼ���������ƿ
��5������������Ҳ�����Ȼ���(ClSO3H)�ֽ��ã��÷�Ӧ�Ļ�ѧ����ʽΪ��2ClSO3H��H2SO4+SO2Cl2���˷����õ��IJ�Ʒ�л�������ᡣ
�ٴӷֽ�����з���������ȵ�ʵ���������Ϊ__________________��
�����ʵ�鷽�������Ȼ���ֽ���ȡ�����Ȳ�Ʒ�л������ᣬ���з����������ǣ�_____(����ĸ��
A.ȡ��Ʒ����ˮ���μ���ɫʯ����Һ��죺��ȡ������Һ������BaCl2��Һ������ɫ������˵������H2SO4��
B.ȡ��Ʒ�ڸ��������¼�������ȫ��Ӧ����ȴ��ֱ�Ӽ�BaCl2��Һ���а�ɫ�������ٵμ���ɫʯ����Һ��죬˵������H2SO4��
��6��Li��SOCl2��ؿ����������������õ�صĵ缫���Ϸֱ�Ϊ﮺�̼���������Һ��LiAlCl4��SOCl2����ص��ܷ�Ӧ�ɱ�ʾΪ��4Li��2SOCl2===4LiCl��S��SO2��
��д���õ�����������ĵ缫��Ӧʽ_______________________________
����װ�õ�ر�������ˮ�������������½��У�ԭ����__________________