��Ŀ����
����Ŀ������P(s)��Cl2(g)������Ӧ����PCl3(g)��PCl5(g)����Ӧ���̺�������ϵ��ͼ��ʾ(ͼ�е���H��ʾ����1mol���������)��
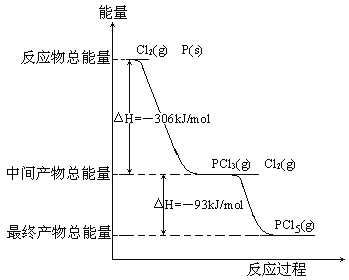
������ͼ�ش��������⣺
(1)P��Cl2��Ӧ����PCl3���Ȼ�ѧ����ʽ_________________________________��
(2)PCl5�ֽ��PCl3��Cl2���Ȼ�ѧ����ʽ_________________________________�������ֽⷴӦ��һ�����淴Ӧ���¶�T1ʱ�����ܱ������м���0.80molPCl5����Ӧ�ﵽƽ��ʱPCl5��ʣ0.60mol����ֽ�����1����_________������Ӧ�¶���T1���ߵ�T2��ƽ��ʱPCl5�ķֽ���Ϊ��2����2_______��1(��������������С��������������)��
(3)��ҵ���Ʊ�PCl5ͨ�����������У��Ƚ�P��Cl2��Ӧ�����м����PCl3��Ȼ���£��ٺ�Cl2��Ӧ����PCl5��ԭ����________________________________________��
(4)P��Cl2��������Ӧ����1molPCl5����H3��_________��P��Cl2һ����Ӧ����1molPCl5����H4______��H3(��������������С��������������)��
(5)PCl5������ˮ��ַ�Ӧ���������������ᣬ�仯ѧ����ʽ��______________________________��
���𰸡���1��P(s)��Cl2(g)===PCl3(g)����H����306kJ��mol��1��
(2��PCl5(g)===PCl3(g)��Cl2(g)����H��93kJ��mol��1��25�������ڡ�
(3��������Ӧ��Ϊ���ȷ�Ӧ�������¶���������߲��ʣ���ֹ����ֽ⡣
(4����399kJ��mol��1�����ڡ�
(5��PCl5��4H2O===H3PO4��5HCl��
��������
��1����ͼ���Կ�����1molP��Cl2����ȫȼ�շų�������Ϊ306kJ��mol��1������P��Cl2��Ӧ����PCl3���Ȼ�ѧ��Ӧ����ʽΪP(s)��Cl2(g)===PCl3(g)����H����306kJ��mol��1��
(2���м����PCl3��δ��ȫ��Ӧ��Cl2���������������ղ���PCl5������������H����93kJ��mol��1������PCl5(g)===PCl3(g)��Cl2(g)����H��93kJ��mol��1���ֽ�����1����100����25�������Ȼ�ѧ��Ӧ����ʽ��֪���˷�Ӧ������ӦΪ���ȷ�Ӧ�����������¶ȣ�ƽ��������Ӧ�����ƶ���PCl5�ķֽ�����������2����1��
(3����ͼ��֪��P��Cl2��Ӧ����PCl3��PCl3��Cl2��һ����Ӧ����PCl5�����Ƿ��ȷ�Ӧ�������������ҵڶ��������¶ȣ�������PCl5�����ɣ���ֹPCl5�ķֽ⡣
(4���ɸ�˹���ɿ�֪��һ����������PCl5����������PCl5������ЧӦ��ȣ�����H3����399kJ��mol��1��
(5��PCl5��ˮ��Ӧ����H3PO4��HCl����ѧ����ʽΪ��PCl5��4H2O===H3PO4��5HCl��

����Ŀ���Իش����и��⣺
��1����ͼ1��ʾ��NO2��CO��Ӧ����CO2��NO�����������仯ʾ��ͼ����д��NO2��CO��Ӧ���Ȼ�ѧ����ʽ�� ______________________________��
��2����ѧ��Ӧ���ʱ��뷴Ӧ���������ļ����йء�
����֪��H2(g)��Cl2(g)===2HCl(g) ��H����185 kJ��mol��1
����գ�
���ۼ� | H��H | Cl��Cl | H��Cl |
����/(kJ��mol��1) | 436 | 247 | ________ |
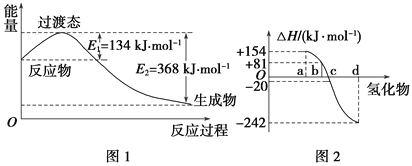
��ͼ2�б�ʾ����Ԫ���������������������⻯��ʱ���ʱ����ݣ������ʱ����ݿ�ȷ��a��b��c��d�ֱ��������Ԫ�أ���д��������������ѧ��״̬�£������ֽⷴӦ���Ȼ�ѧ����ʽ�� ___________________________________��