��Ŀ����
25��ʱ����������ĵ��볣�����£�
25��ʱ������˵����ȷ���ǣ� ��
A�������ʵ���Ũ�ȵĸ���ҺpH��ϵΪ��pH(CH3COONa)> pH(Na2S) > pH(NaCN)
B��a mol/LHCN��Һ��b mol/LNaOH��Һ�������ϣ�������Һ��c(Na+)>c(CN-)����aһ������b
C��NaHS��Na2S�Ļ����Һ�У�һ������c(Na+)��c(H+)��c(OH-)��c(HS-)��2c(S2-)
D��ijŨ�ȵ�NaCN��Һ��pH��d����������ˮ�������c(OH-)��10-dmol/L
| ����Ļ�ѧʽ | CH3COOH | HCN | H2S |
| ���볣����25�棩 | 1.8��10-5 | 4.9��10-10 | K1��1.3��10-7 K2��7.1��10-15 |
25��ʱ������˵����ȷ���ǣ� ��
A�������ʵ���Ũ�ȵĸ���ҺpH��ϵΪ��pH(CH3COONa)> pH(Na2S) > pH(NaCN)
B��a mol/LHCN��Һ��b mol/LNaOH��Һ�������ϣ�������Һ��c(Na+)>c(CN-)����aһ������b
C��NaHS��Na2S�Ļ����Һ�У�һ������c(Na+)��c(H+)��c(OH-)��c(HS-)��2c(S2-)
D��ijŨ�ȵ�NaCN��Һ��pH��d����������ˮ�������c(OH-)��10-dmol/L
C
���������A��Na2Sˮ����S2-+H2O
 HS-+OH-Ϊ��ˮ��̶���K2��أ���ˮ��̶�Na2S> NaCN >CH3COONa��pHҲ����ͬ��ϵ������B����a=b������Һǡ����ȫ��Ӧ����NaCN����Һ��c(Na+)>c(CN-)������C������غ�ʽ����ȷ��D��NaCNˮ����Һ�ʼ��ԣ�c(H+)��10-dmol/L��c(OH-)��10d��14mol/L������
HS-+OH-Ϊ��ˮ��̶���K2��أ���ˮ��̶�Na2S> NaCN >CH3COONa��pHҲ����ͬ��ϵ������B����a=b������Һǡ����ȫ��Ӧ����NaCN����Һ��c(Na+)>c(CN-)������C������غ�ʽ����ȷ��D��NaCNˮ����Һ�ʼ��ԣ�c(H+)��10-dmol/L��c(OH-)��10d��14mol/L������
��ϰ��ϵ�д�
 Сѧ�̲���ȫ���ϵ�д�
Сѧ�̲���ȫ���ϵ�д�
�����Ŀ

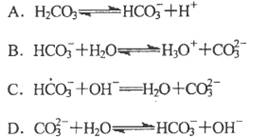
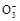
 H++C
H++C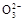
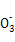 )+2c(R
)+2c(R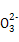 )
)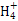 ):�٣��ۣ���
):�٣��ۣ��� S2-��H3O+
S2-��H3O+