��Ŀ����
���Ų��Ͽ�ѧ�ķ�չ�����������仯����õ���Խ��Խ�㷺��Ӧ�ã�������Ϊ���Ͻ�ά���ء�����ҵ�ϻ��շϷ�����������V2O5��VOSO4��K2SO4��SiO2���з�����Ҫ�������£�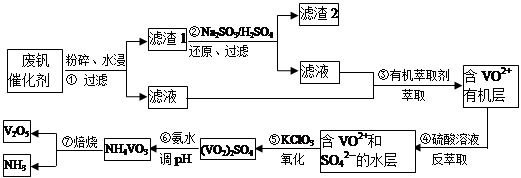
��֪����1��V2O5��NH4VO3��Ϊ�����VOSO4��(VO2)2SO4��Ϊ�����
��2�� 2VO2++H2C2O4+2H+ = 2VO2+ + 2CO2��+ 2H2O
�ش��������⣺
��1�������ǰ�������Ŀ����_________________________��
��2��������з�����Ӧ�����ӷ���ʽΪ__________________________��
��3������۵ı仯���̿ɼ�Ϊ(HA��ʾ�л���ȡ��)��
VOSO4 (ˮ��)+ 2HA���л��㣩 VOA2(�л��㣩+ H2SO4(ˮ��)��������п�ѡ������������ȡ��ԭ����_____________��
VOA2(�л��㣩+ H2SO4(ˮ��)��������п�ѡ������������ȡ��ԭ����_____________��
��4���������ữ��H2C2O4��Һ�ζ�(VO2)2SO4��Һ���Բⶨ�����ݺ���Һ�к������IJ���Ϊ��ȡ10.0mL0.1mol/LH2C2O4��Һ����ƿ�У�����ָ�����������Һʢ���ڵζ����У��ζ����յ�ʱ�����Ĵ���Һ�����Ϊ10.0mL���ɴ˿�֪(VO2)2SO4��Һ��Ԫ�صĺ���Ϊ_________g/L��
��5��V2O5���ý���(��Ca��Al)�Ȼ�ԭ����÷�����������Ȼ�ԭ�Ƶ÷��Ļ�ѧ����ʽΪ_______________��
��1�������Һ�Ӵ�������ӿ�������ʣ���߽����ʣ�2�֣�
��2��V2O5+ SO32��+4H+=2VO2+ + SO42��+2H2O��2�֣�
��3���������ᣬ��ʹƽ��������У�ʹVOSO4����ˮ�㣻��2�֣�
��4��10.2g/L ��2�֣�
��5��3V2O5+10Al 6V+5Al2O3��2�֣�
6V+5Al2O3��2�֣�
���������������1��������巴Ӧ����������Һ�Ӵ�������ӿ�������ʣ���߽����ʡ�
��2������������л�ԭ�ԣ����������£��ܱ���������������������������ӣ����ӷ�Ӧ����ʽΪ��V2O5+ SO32��+4H+=2VO2+ + SO42��+2H2O
��3������H2SO4��H2SO4Ũ��������ۻ�ѧƽ���������ƶ���ʹVOSO4����ˮ�㡣
��4��������Ŀ������Ϣ��2VO2+ ~ H2C2O4����֪(VO2)2SO4��Һ��Ԫ�صĺ���Ϊ��0.1mol/L��0.01L��2��51g/mol��0.01L= 10.2g/L��
��5������������������Ӧ���ɷ�������������ѧ����ʽΪ��3V2O5+10Al 6V+5Al2O3
6V+5Al2O3
���㣺���⿼�黯ѧ�������̵ķ���������ʽ����д����ѧ���㡣

 �����ҵ���������ͯ������ϵ�д�
�����ҵ���������ͯ������ϵ�д�����þ����Ҫ�ɷ�ΪMgCO3������ȼ��������þ�Ĺ����������£�
��1����������ͼ���Եó��Ľ���Ϊ �� ��
ͼ1 25��ʱMgOˮ����ʱ��仯X����������ͼ 
ͼ2 90��ʱMgOˮ����ʱ��仯X����������ͼ
��2��ˮ����ӦMgO+H2O = Mg(OH)2���Է����е�ԭ���� ��
��3�����Ԫ�������ɺͱ�1��֪�����������������ȷֽ�Ĺ����� ����дһ�����ɣ�
��1 ��������Ԫ�صĽ������������ȷֽ��¶�/��
| LiOH | NaOH | KOH | Al(OH)3 | Mg(OH)2 | Ca(OH)2 | Ba(OH)2 |
| 924 | ���ֽ� | ���ֽ� | 140 | 258 | 390 | 700 |
��4����֪�Ȼ�ѧ����ʽ��Mg(OH)2 (s) =" MgO" (s)+H2O (g) ��H =" 81.5" kJ��mol��1
��Mg(OH)2����ȼ���õ���Ҫԭ���� ��
���볣��±ϵ�����������飩���л���ϵ����������������ȼ����ȣ�Mg(OH)2��ȼ�����ŵ��� ��
(14��)ﯲ�ҵ�Ǽ��з�չDZ����ǰ�������˲�ҵ���(Zr)Ԫ���Ǻ˷�Ӧ��ȼ�ϰ��İ������ϣ��������(ZrO2)�����������������մɡ��ҹ��зḻ���Ӣʯ(ZrSiO4)����Al2O3��SiO2��Fe2O3�����ʣ����������֮һ���£�
�Իش��������⣺
(1)д�����������и��������ķ�Ӧ����ʽ(̼ת����CO)��_______________________________________________��
(2)д��ZrOCl2��8H2O��900 ������ZrO2�ķ�Ӧ����ʽ�� ______________ ______________________________________________��
(3)���ڶ�����������մɺ�ﯺϽ��˵������ȷ����________(��ѡ)��
| A��������������մ����������ǽ������� |
| B��1 nm��10��10m |
| C��ﯺϽ��Ӳ�ȱȴ��Ҫ�� |
| D���ձ������˵�վ�ı�ը��������ﯺϽ��ڸ�������ˮ������Ӧ���������� |
(4)һ������ȼ�ϵ�أ�һ��ͨ���������һ��ͨ�붡�飻������Dz���������(Y2O3)�Ķ������(ZrO2)���壬������״̬���ܴ���O2���������ڵ�����У�O2����________(���������)���ƶ�����������缫��ӦΪ__________________�������缫��ӦΪ________________________________��
��ҵ����������������Fe2+��Fe3+�������μ�����CaO��MgO���Ʊ��ߵ���������(Fe2O3 )�ͻ���(NH4)2SO4�����������������£�
�ش��������⣺
��1���ڷ����ܽ����ʱ��Ϊ�˼��ٷ����ܽ�Ĵ�ʩ�ǣ�___________________��__________________����д���㣩
��2������A��һ��������
�ٹ�ҵ�����ѡ�� ������ţ�
| A������ | B��Cl2 | C��MnO2 | D��H2O2�� |
��д��A���뷴Ӧ�����ӷ���ʽΪ__________________________________________
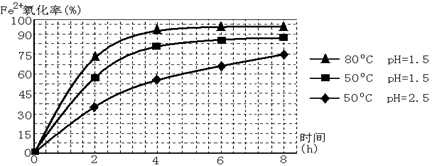
��3������ͼ�й����ݣ�����Ϊ��ҵ����������ʱӦ���Ƶ�������__________________��
��4����炙�������Һ(��Fe3+)�м�����ҺB��pHΪ5ʱ������������д���������������ӷ���ʽ��_______________________________________________________��
��5��д��炙���������������������ˮ�У������м������Ba(OH)2��Һ��������Ӧ�����ӷ���ʽΪ��_____________________________________________________
��6���������õ�(NH4)2SO4������ܺ��е������ǣ�___________________________
[��ѧ��ѡ��ѧ�뼼��]��15�֣�
��ˮ����þ(MgSO4��7H2O)��ӡȾ����ֽ��ҽҩ�ȹ�ҵ�϶��й㷺��Ӧ�ã����û�����������ɰ�ķ�������þ�����ȡ��ˮ����þ����þ�����Ҫ�ɷ���MgCO3����������������(MgO��SiO2��Fe2O3��FeO��CaO��Al2O3��MnO�ȣ���
��1 ����������������������ʽ��ȫ����ʱ��Һ��pH
| ������ | Al(OH)3 | Fe(OH)3 | Fe(OH)2 | Mn(OH)2 | Mg(OH)2 |
| pHֵ | 5.2 | 3.2 | 9.7 | 10.4 | 11.2 |
��2 �����ε��ܽ��(��λΪg��100gˮ)
| �¶�/�� | 10 | 30 | 40 | 50 | 60 |
| CaSO4 | 0.19 | 0.21 | 0.21 | 0.20 | 0.19 |
| MgSO4��7H2O | 30.9 | 35.5 | 40.8 | 45.6 | / |
��þ����ȡ��ˮ����þ�Ĺ����������£�

������������ͼ���ο�����pH���ݺ��ܽ�����ݣ��Իش��������⣺
��1������I����Һ�м�����þ�࣬������Һ��pH��5��6���ټ���NaClO��Һ������У�����Һ�е� Mn2+������MnO2����Ӧ�����ӷ�Ӧ����ʽΪ ��������е���ҪĿ�� �� ��
��2������B�г�MnO2��SiO2����� (�ѧʽ)�����ʡ�
��3��������ˢ�����Һ���Ƿ���Fe3+��ʵ�鷽���� ��
��4������C�Ļ�ѧʽ�� ������III����ȹ��˵�������
���еĻ�����Ⱦ�ǵ�ǰͻ���Ļ������⡣��ͼA��ʾ��X������ij��ҵ����Y������a�������Σ������зḻ�Ļ�������Դ��d�������Σ������ж����ҵ������Ϊԭ�ϡ�����ij��Y�л����ּ��վ��X��ˮ�ʼ��Ľ�������Ƴɼ�ͼ��ͼB��ʾ������˵������ȷ����( )
| A�����X����Ⱦ����Ҫ��ȾԴ����ֲܷ���bc�� |
| B����ҵ������ˮ�����������ȿ��������X����Ⱦ����ȾԴ |
| C��d����Ⱦ�̶ȼ�С��������ijЩ��Ⱦ������Ӽ䷢����Ӧ���ɳ������� |
| D���ó��н������᳧ʱ���ۺϿ����������ѡַ��b������� |