��Ŀ����
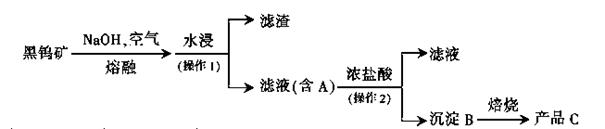
��1��������һ����Ҫ�Ļ���ԭ�ϡ�Ŀǰ�Ƽҵ��Ҫ�С�������͡������Ƽ���������Ƽ�����ֹ��ա�
�١��������������CaCl2�����д���ù����в���CaCl2�Ļ�ѧ����ʽ��_________________________________________________��
��д���������Ƽ���йط�Ӧ�Ļ�ѧ����ʽ_________________ _�� ��
��CO2���Ƽҵ����Ҫԭ�ϣ��������Ƽ���롰�������CO2����Դ�кβ�ͬ��________________________________________��
��2��������ҵ�Դٽ����ú���ᷢչ������Ҫ���á�
������ʱ������衢�̺�����Ŀ����_______________________________��
�ڲ���ֺ��е�CrԪ���������ֹ��̵�����__ __���ǰ�������롣
�����������������У�β�������е���Ҫ��Ⱦ����________���ӻ����;��ýǶȿ��ǣ�����β��������������_________��
��1����2NH4Cl��Ca(OH)2 2NH3����CaCl2��2H2O ��2�֣�
2NH3����CaCl2��2H2O ��2�֣�
��NH3��CO2��H2O��NaCl�����ͣ���NaHCO3����NH4Cl ��2�֣�
2NaHCO3 Na2CO3��CO2����H2O ��2�֣�
Na2CO3��CO2����H2O ��2�֣�
����д�ܷ�Ӧ����ʽ��2NaCl��2NH3��CO2��H2O��Na2CO3��2NH4Cl��
�ۡ������CO2��Դ��ʯ��ʯ���գ��������Ƽ��CO2��Դ�ںϳɰ���ҵ�ķ�������2�֣�
��2���������͵����ֵijɷ� ��2�֣�
�ں� ��1�֣�
��CO ��2�֣� ȼ�ϣ���ԭ���� ��2�֣�
���������������1�� �ٰ����Ϊ���հ���ʹʯ�����븱�����Ȼ�立�Ӧ���Ӷ���������CaCl2������2NH4Cl + Ca(OH)2=CaCl2+2H2O+2NH3�����������Ƽ����Ҫ��ѧ��ӦΪ��
NaCl(����)+CO2+NH3+H2O=NaHCO3��+NH4Cl 2NaHCO3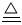 Na2CO3+H2O+CO2��
Na2CO3+H2O+CO2��
�۰����CO2��Դ��ʯ��ʯ�����գ��������Ƽ����CO2��Դ�ںϳɰ���ҵ�ķ�����
��2��������ʱ������衢�̺�����Ҫ�ǿ��������͵����ֵijɷ֣��� ��ΪCr�ױ�������Ϊ��ֹCr������������ֺ��е�CrԪ���������ֹ��̵�������������ǰ����Cr���γ�¯������ȥ�������������������У�CO����Ҫ�Ļ�ԭ������β�������е���Ҫ��Ⱦ����CO��һ����̼�������ж������������д�����
���㣺���黯ѧ�뼼����

�ҹ��зḻ����Ȼ����Դ������Ȼ��Ϊԭ�Ϻϳ����ص���Ҫ������ͼ��ʾ(ͼ��ijЩת�����輰������δ�г�):
����д���пհ�:
(1)��֪0.5 mol�����0.5 molˮ������t�桢p kPaʱ,��ȫ��Ӧ����һ����̼������(�ϳ���),������a kJ�������÷�Ӧ���Ȼ�ѧ����ʽ����������������
(2)����������,��ҵ�Ϸ���H2��CO2�����ķ�������������
| A���������ͨ������������Һ,������Һ�м������� |
| B���������ѹ��ȴ,ʹCO2Һ�� |
| C��������ð�ˮϴ�� |
| D���������ͨ��ʯ�ҽ���,Ȼ��������չ��� |
(4)������������Դ����������߾���Ч��,Ҳ�Ƕ���ᡢ��ȫ���ฺ��ı���,�����߶κͼ�ͷ����ͼ�е���������������Դ�����
�ҹ����������ձ��ȹ��Ҷ������Ƴ�һ���մɲ��ͻ������ֲ��ͻ��ķ���������������������һ�������Ҳ��״��ȵIJ���������ģ����ֲ�����(����)��
| A���������մ� | B���������մ� |
| C�����ά | D�������� |
���й�ҵ�����У������ʵ����ʵ�����Ĺ�ϵʽ����ȷ���ǣ� ��
| A����Ư�ۣ�2Cl2��Ca(ClO)2 |
| B����H2SO4��FeS2��2H2SO4 |
| C���ϳɰ���C��H2��2/3NH3 |
| D����HNO3��NH3��HNO3 |


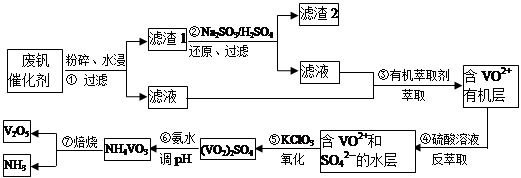
 VOA2(�л��㣩+ H2SO4(ˮ��)��������п�ѡ������������ȡ��ԭ����_____________��
VOA2(�л��㣩+ H2SO4(ˮ��)��������п�ѡ������������ȡ��ԭ����_____________��
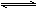 Na2S2O5��H2O�ȶಽ��Ӧ��
Na2S2O5��H2O�ȶಽ��Ӧ��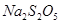 ��������ʳƷƯ�������Ʊ������������£�
��������ʳƷƯ�������Ʊ������������£�
 �ȶಽ��Ӧ��
�ȶಽ��Ӧ��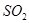 �������ӷ���ʽΪ___________��
�������ӷ���ʽΪ___________��