��Ŀ����
��10�֣���ҵ����MnO2��KOHΪԭ����ȡ������أ���Ҫ�������̷��������С���һ����MnO2����KOH���飬��Ͼ��ȣ��ڿ����м������ۻ������������裬��ȡK2MnO4���ڶ�����K2MnO4��Ũ��Һ���е�⣬��ȡKMnO4���Իش��������⣺
��1����ȡK2MnO4��MnO2����һ������ԭ��Ӧ��_____________�������������ԭ���������������Ŀ����________________________________��
��2�����K2MnO4��Ũ��Һʱ�����������ĵ缫��ӦʽΪ��
������_______________________,
������_________________________��
����ܵķ�Ӧ����ʽ��_________________________��
��1����ȡK2MnO4��MnO2����һ������ԭ��Ӧ��_____________�������������ԭ���������������Ŀ����________________________________��
��2�����K2MnO4��Ũ��Һʱ�����������ĵ缫��ӦʽΪ��
������_______________________,
������_________________________��
����ܵķ�Ӧ����ʽ��_________________________��
��10�֣�ÿ��2�֣���1����ԭ�����������ֽӴ���ʹMnO2������ȫ
��2��2H++2e-==H2����MnO42��-e-="==" MnO4-,2K2MnO4+2H2O==2KMnO4+2KOH+ H2��
��2��2H++2e-==H2����MnO42��-e-="==" MnO4-,2K2MnO4+2H2O==2KMnO4+2KOH+ H2��
��

��ϰ��ϵ�д�
 ���¿쳵����������ϵ�д�
���¿쳵����������ϵ�д�
�����Ŀ
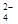
 2Fe+3CO2�������������ԭ��Ӧ�У��������ͻ�ԭ�������ʵ���֮��Ϊ( )
2Fe+3CO2�������������ԭ��Ӧ�У��������ͻ�ԭ�������ʵ���֮��Ϊ( )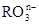 ��6I����6H����R����3I2��3H2O��n��_____��RԪ����
��6I����6H����R����3I2��3H2O��n��_____��RԪ����