��Ŀ����
6�����أ�A����ʹ��ˮ��ɫ��������̼������Һ��Ӧ�ų�CO2��A��CnH2n+1��Ӧ�����ɷ���ʽΪCn+3H2n+4O2�������ش��������⣺��1��A����ṹ��ʽΪCH2=CH-COOH�����������ŵ�����Ϊ��̼̼˫�����Ȼ���
��2����֪��̼̼˫�����л�����±���������ӳɷ�Ӧʱ����ԭ�����Ǽӵ�˫���к���϶��̼ԭ���ϣ����ι���A��HBr�����ӳɷ�Ӧ���ɵ�����B�Ľṹ��ʽΪ
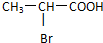 ��
����3��д��A���Ҵ�������Ӧ�Ļ�ѧ����ʽ
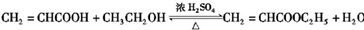 ���÷�Ӧ�ķ�Ӧ����Ϊ������Ӧ����ȡ����Ӧ����
���÷�Ӧ�ķ�Ӧ����Ϊ������Ӧ����ȡ����Ӧ������4����֪B��NaOH��Һ����ȡ����Ӧ�����������ữ������C�Ľṹ��ʽΪ��
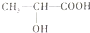 ����д��C�����Na��Ӧ�Ļ�ѧ����ʽΪ��
����д��C�����Na��Ӧ�Ļ�ѧ����ʽΪ��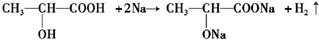 ��
��
���� ��1���л���A����ʹ��ˮ��ɫ�����в����ͼ�������̼������Һ��Ӧ�ų�CO2������-COOH��A��CnH2n+1OH��Ӧ���ɷ���ʽΪCn+3H2n+4O2�����������ΪһԪ�����л���A�ķ���ʽΪ��Cn+3H2n+4O2+H2O-CnH2n+1OH=C3H4O2����A�Ľṹ��ʽΪCH2=CH-COOH��
��2������Ŀ��Ϣ��֪��HBr����ԭ�Ӽӵ���ԭ�ӽ϶�IJ�����̼ԭ���ϣ�Brԭ�Ӽӵ�����ԭ�ӽ��ٵIJ�����̼ԭ���ϣ��ݴ��ж�B�Ľṹ��ʽ��
��3��A�����к����Ȼ����ܹ��Ҵ�����������Ӧ���ݴ�д����Ӧ�Ļ�ѧ����ʽ��
��4���ǻ����Ȼ������Ʒ�Ӧ�����������ݴ�д����Ӧ�Ļ�ѧ����ʽ��
��� �⣺��1��A��CnH2n+1OH��Ӧ���ɷ���ʽΪCn+3H2n+4O2�����������ΪһԪ�����л���A�ķ���ʽΪ��Cn+3H2n+4O2+H2O-CnH2n+1OH=C3H4O2���л���A����ʹ��ˮ��ɫ�����в����ͼ�������̼������Һ��Ӧ�ų�CO2������-COOH����A�Ľṹ��ʽΪCH2=CH-COOH��
�ʴ�Ϊ��CH2=CH-COOH��̼̼˫�����Ȼ���
��2������Ŀ��Ϣ��֪��HBr����ԭ�Ӽӵ���ԭ�ӽ϶�IJ�����̼ԭ���ϣ�Brԭ�Ӽӵ�����ԭ�ӽ��ٵIJ�����̼ԭ���ϣ���B�Ľṹ��ʽΪ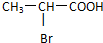 ��
��
�ʴ�Ϊ��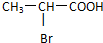 ��
��
��3��A�����к����Ȼ����ܹ����Ҵ�����������Ӧ����Ӧ�Ļ�ѧ����ʽΪ��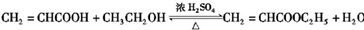 ���÷�ӦΪ������Ӧ��ȡ����Ӧ��
���÷�ӦΪ������Ӧ��ȡ����Ӧ��
�ʴ�Ϊ��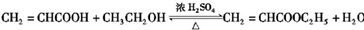 ��������Ӧ����ȡ����Ӧ����
��������Ӧ����ȡ����Ӧ����
��4�� �����еĴ��ǻ����Ȼ����ܹ����Ʒ�Ӧ������������Ӧ�ķ���ʽΪ��
�����еĴ��ǻ����Ȼ����ܹ����Ʒ�Ӧ������������Ӧ�ķ���ʽΪ��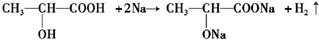 ��
��
�ʴ�Ϊ��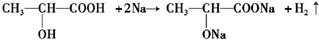 ��
��
���� ���⿼���л������ʽ���ṹ��ʽ��ȷ������Ŀ�Ѷ��еȣ����չ����ŵ�������ת���ǹؼ������ضԻ���֪ʶ�Ĺ��̣�������ѧ���ķ������������Ӧ�û���֪ʶ��������

| A�� | v��A2��=0.8mol•L-1•s-1 | B�� | v��A2��=60mol•L-1•min-1 | ||
| C�� | v��AB3��=1.0mol•L-1•s-1 | D�� | v��B2��=1.2mol•L-1•s-1 |
| A�� | �Լ�AΪʯ���飬�۸�����������Ա��� | |
| B�� | �������м�����Լ�Ϊ���� | |
| C�� | ������ȡþ�Ĺ�����û���漰������ԭ��Ӧ | |
| D�� | �������������� |
| A�� | ��������������֯�з�����������ʱ������ת��Ϊ��ѧ�� | |
| B�� | ��ɫֲ����й������ʱ��̫����ת��Ϊ������ | |
| C�� | úȼ��ʱ����ѧ����Ҫת��Ϊ���� | |
| D�� | ���ˮ��������������ʱ������ת��Ϊ��ѧ�� |
| A�� | ���ӻ������п��Ժ��й��ۼ��������ۻ�������һ���������Ӽ� | |
| B�� | ��̬���ʷ�����һ�����ڹ��ۼ� | |
| C�� | ��Ԫ�ؼ�������Ԫ���γɹ��ۼ�Ҳ������Ԫ���γ����Ӽ� | |
| D�� | ����Ԫ�غͷǽ���Ԫ���γɵĻ����ﲻһ�������ӻ����� |
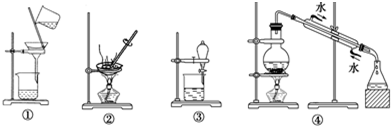
| A�� | ����Na2CO3��Һ��CH3COOC2H5��ѡ�� | |
| B�� | ��CCl4��ȡ��ˮ�еĵ⣬ѡ�ۺ͢� | |
| C�� | ��ȥ�Ȼ����е���������ع��壬ѡ�ں͢� | |
| D�� | ģ��ѪҺ����ѡ�� |
| A�� | FeCl2��ͨ�����Ϸ�Ӧһ���Ƶ� | |
| B�� | ������ϡ��������������̡�����ͭ����ԭ�������ֺ�ɫ��ĩ | |
| C�� | ���ý�MgSO4��Һֱ�����ɵķ��������Ʊ�MgSO4���� | |
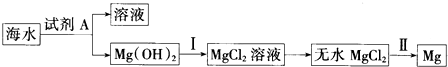
| D�� | ��ͨ���������MgCl2����ȡMg����Ҳ��ͨ��������ڵ�AlCl3����ȡAl |
| A�� | ̼ˮ������ | B�� | ���� | C�� | ���� | D�� | ̼�⻯���� |
| A�� | �������֡��ơ�ʳ�� | B�� | ���͡���֬����ȩ��֬ | ||
| C�� | ʯ̿�ᡢ���ᡢ������ | D�� | ���͡��Ҵ��ơ��ȷ� |