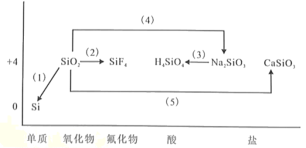
��Ŀ����
����Ŀ��������һ��̽��ī�������������ī��������֬��̿�ڡ���ɼ����ŷ�(��������)����ɡ�
(1)��һ����ī���м���������ϡ�����������Թ��л���ѹ���������Թܵײ������������������___________��
(2)ȡ(1)��ˮ�㲿����Һ������Һ��װ���Թܣ�һ�Թ��м�������![]() ��Һ������_________��֤����Fe3������һ�Թ��м������軯����Һ����������______��֤����Fe2����
��Һ������_________��֤����Fe3������һ�Թ��м������軯����Һ����������______��֤����Fe2����
������������ʵ�鷽�����ⶨī������Ԫ�ص�����������ȡ![]() ī�۾���һϵ�в��������Ƴ�
ī�۾���һϵ�в��������Ƴ�![]() ��Һ(��Һ����Ԫ��ȫ��ת��ΪFe3��)��ȡ��
��Һ(��Һ����Ԫ��ȫ��ת��ΪFe3��)��ȡ��![]() ��Һ����
��Һ����![]() ��
��![]() ��Һ�ζ���
��Һ�ζ���
(1)�ζ������У�ѡ���ָʾ����______��
a.������Һ b. ![]() ��Һ c.
��Һ c.![]() ��Һ d.
��Һ d.![]() ��Һ
��Һ
(2)���ζ�����������![]() ��Һ
��Һ![]() ����ī����������������Ϊ______(�ٷ���������λ����)��
����ī����������������Ϊ______(�ٷ���������λ����)��
�����������˽�![]() �Ĺ�ҵ�Ʊ�����ӡ��ʹ�õ�ī�۵���Ҫ�ɷ���
�Ĺ�ҵ�Ʊ�����ӡ��ʹ�õ�ī�۵���Ҫ�ɷ���![]() ����ͼ�����������������Ʊ��ߴ�
����ͼ�����������������Ʊ��ߴ�![]() �Ĺ������̡�
�Ĺ������̡�

��֪��
��![]() ��
��
��![]() ��ˮ�п��ܣ����Ҵ������ܣ�
��ˮ�п��ܣ����Ҵ������ܣ�
��![]() ���Ҵ���ˮ�Ļ���ܼ��е��ܽ�����Ҵ���������ı仯������ͼ��ʾ��
���Ҵ���ˮ�Ļ���ܼ��е��ܽ�����Ҵ���������ı仯������ͼ��ʾ��

�ش��������⣺
(1)�ٽ���Һ�к��еĽ���������Ϊ______________���������м�����м��Ŀ����_________��
(2)�������![]() �����������ŵ���_______��
�����������ŵ���_______��
(3)������ݺ�һ����Ҫ�����þ������ϴ�Ӻ��ﴦ������˵��ѡ���Ҵ���ˮ�Ļ��Һ��Ϊϴ��Һ������___________��
���𰸡����� ���ɫ���� ��ɫ���� b 66% Fe2�� ��ֹFe2�������� ���������ʡ�����Ⱦ ϴȥ![]() ������
������![]() ���ܽ�
���ܽ�
��������
������һ����1����һ����ī���м���������ϡ�����������Թ��л���ѹ���������Թܵײ������������ܽ⣬̼�����ʲ��ܣ���������������ǹ��ˡ��ʴ�Ϊ�����ˣ�
��2��ȡ��1����ˮ�㲿����Һ������Һ��װ���Թܣ�һ�Թ��м�������![]() ��Һ�����ֺ��ɫ����[Fe3����3OH��=Fe(OH)3��]��֤����Fe3������һ�Թ��м������軯����Һ������������ɫ����������Fe3[Fe(CN)6]2����ɫ������֤����Fe2�����ʴ�Ϊ�����ɫ��������ɫ������
��Һ�����ֺ��ɫ����[Fe3����3OH��=Fe(OH)3��]��֤����Fe3������һ�Թ��м������軯����Һ������������ɫ����������Fe3[Fe(CN)6]2����ɫ������֤����Fe2�����ʴ�Ϊ�����ɫ��������ɫ������
�����������1���ζ���ԭ��Ϊ2Fe3����2I��=2Fe2����I2���ζ������У���![]() ��Һ��ָʾ������ɫ��ȥʱ��ֹͣ�ζ����ʴ�Ϊ��b��
��Һ��ָʾ������ɫ��ȥʱ��ֹͣ�ζ����ʴ�Ϊ��b��
��2�����ζ�����������![]() ��Һ
��Һ![]() ����ī����������������Ϊ
����ī����������������Ϊ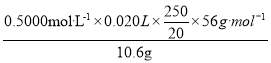 ��100%= 66%��
��100%= 66%��
�ʴ�Ϊ��66%��
������������1���������������������ᣬ����H2S�������ӻ�ԭ����Fe�ɷ�������������������Һ�к��еĽ���������ΪFe2�����������м�����м��Ŀ���Ƿ�ֹFe2�����������ʴ�Ϊ��Fe2������ֹFe2����������
��2��˫��ˮ����ɫ����������Ӧ������ˮ���������![]() �����������ŵ��Dz��������ʡ�����Ⱦ���ʴ�Ϊ�����������ʡ�����Ⱦ��
�����������ŵ��Dz��������ʡ�����Ⱦ���ʴ�Ϊ�����������ʡ�����Ⱦ��
��3��������Ŀ�ṩ����Ϣ�ڢۣ�������ݺ����þ������ϴ�Ӻ��ﴦ����ѡ���Ҵ���ˮ�Ļ��Һ��Ϊϴ��Һ������ϴȥ![]() ������
������![]() ���ܽ⡣�ʴ�Ϊ��ϴȥ
���ܽ⡣�ʴ�Ϊ��ϴȥ![]() ������
������![]() ���ܽ⡣
���ܽ⡣

 ÿ��10���ӿ�����������������ϵ�д�
ÿ��10���ӿ�����������������ϵ�д�����Ŀ����֪����1mol������к���2molSi��Si����
��Si(s)��O2(g)===SiO2(g) ��H���䷴Ӧ�����������仯��ͼ��ʾ��
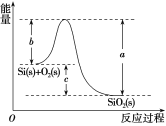
��
��ѧ�� | Si��O | O=O | Si��Si |
�Ͽ�1 mol���ۼ���������/kJ | 460 | 500 | 176 |
����˵������ȷ���ǣ� ��
A.������������ǽ���ѧ��ת��Ϊ����B.���������ȶ���С�ڹ���ȶ���
C.��H����988kJ��mol��1D.��H��(a��c)kJ��mol��1