��Ŀ����
����Ŀ����2016������һģ������( PH3)��һ�־綾���壬����õĸ�ЧѬ��ɱ�����Ҳ��һ�ֵ��ӹ�ҵԭ�ϡ�
��1�����ܱ����ַ��õ�����(AlP)Ƭ������ˮ�����ų�PH3���壬�÷�Ӧ�Ļ�ѧ����ʽΪ ��
��2�����÷�ӦPH3+3HgCl2= P(HgCl)3 +3HCl����ȷ�ⶨ����PH3��
��HgCl2��Һ���������磬˵��HgCl2����________________�����������������������������
��ͨ���ⶨ��Һ____________�仯���ɲⶨһ�����������PH3��Ũ�ȡ�
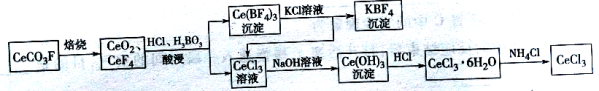
��3��PH3��һ�ֹ�ҵ�Ʒ��漰������ת����ϵ����ͼ��ʾ�� 
������������________________Ԫ�ᡣ
������ӦI���ɵ�n(NaH2PO2):n(Na2HPO3) =3:1ʱ���μӷ�Ӧ��n(P4)��n(NaOH)= ��
��4��һ�����ڴ���PH3���������ռ��ɷ�Ϊ�������80%����ľм�����ɼ���15%������̿2.5%����ʯ�ۣ�����飩2.5%��
��������ƽ�PH3����ΪH3PO4�Ļ�ѧ����ʽΪ________________��
�������е�ˮ�����ɼӿ�PH3���������̣���ԭ�������____________��
��5���ӣ�4���е����ղ������л����������(CaHPO4)�ķ������£�
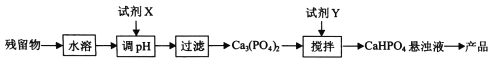
���Լ�xΪ ���ѧʽ����
����֪25��ʱ��H3PO4��Kal=7.5��10-3��Ka2=6.3��10-8��Ka3 =4.4��10-13������Yʱ��Ӧ��������ҺpH 7������>������=������<������ͨ������˵������ ��
���𰸡���1��AlP+3H2O====Al��OH��3+PH3��
��2�������ۣ�����ҺpH��絼�ʡ�
��3���������� 3:10��
��4����PH3+2Ca��ClO��2===H3PO4+2CaCl2�������������ˮ�����Ӵ���ˮ��õ������Ը�ǿ�Ĵ����ᡣ
��5��������
��HPO42-�ĵ��볣����Ka(HPO42-)=4.4��10-13��HPO42-��ˮ�ⳣ��Kh(HPO42-)=Kw/Ka2=1��10-14/6.3��10-8��Ka(HPO42-)��HPO42-��ˮ��̶ȴ��������̶ȣ���Һ�ʼ��ԣ�Ӧ��������ҺpH��7��
��������
�����������1������(AlP)��ˮ������Ӧ������������������PH3���壬�÷�Ӧ�Ļ�ѧ����ʽΪAlP+3H2O====Al��OH��3+PH3��
��2����HgCl2��Һ���������磬˵��HgCl2���ڹ��ۻ���������ݷ�ӦPH3+3HgCl2= P(HgCI)3 +3HCl֪��Ӧ��HCl���ɣ���Һ��pH�͵����Է����仯���ʿ�ͨ���ⶨ��ҺpH��絼�ʱ仯���ɲⶨһ�����������PH3��Ũ�ȡ�
��3������ת����ϵ֪�������ƵĻ�ѧʽΪNa2HPO3�������������ڶ�Ԫ�ᡣ������ӦI���ɵ�n(NaH2PO2):n(Na2HPO3) =3:1ʱ��������Ӧ�Ļ�ѧ����ʽΪ3P4+10NaOH+8H2O==6NaH2PO2+ 2Na2HPO3+4PH3���μӷ�Ӧ��n(P4)��n(NaOH)=3:10��
��4�������������PH3��Ӧ����H3PO4���Ȼ��ƣ���ѧ����ʽΪPH3+2Ca��ClO��2===H3PO4+2CaCl2�� �������е�ˮ�����ɼӿ�PH3���������̣���ԭ������Ǵ��������ˮ�����Ӵ���ˮ��õ������Ը�ǿ�Ĵ����ᡣ
��5������4���е����ղ������к������ᣬ�����������ƻ������ƣ���������ƺ��Ȼ��ơ�������������ᷴӦ����CaHPO4��HPO42-�ĵ��볣����Ka(HPO42-)=4.4��10-13��HPO42-��ˮ�ⳣ��Kh(HPO42-)=Kw/Ka2=1��10-14/6.3��10-8��Ka(HPO42-)��HPO42-��ˮ��̶ȴ��������̶ȣ���Һ�ʼ��ԣ�Ӧ��������ҺpH��7��